Pragmatic Trials for GLP‑1–Based Obesity Treatment in Primary Care
Verified by Sahaj Satani from ImplementMD



The Implementation Gap
GLP-1 receptor agonists represent a landmark advance in obesity pharmacotherapy, yet a vast implementation chasm separates clinical efficacy from population-level impact. The SELECT trial demonstrated 20% cardiovascular risk reduction (HR 0.80; 95% CI: 0.72–0.90) in patients with obesity and established cardiovascular disease (Lincoff et al., 2023), while tirzepatide achieves 15–21% weight reduction in controlled settings. Despite this, only 2.3% of eligible patients receive GLP-1 prescriptions in real-world practice (Kim et al., 2025). Four structural barriers perpetuate this gap: prohibitive cost ($936–$1,349/month list price), restrictive payor policies (only 13 state Medicaid programs cover obesity indications), limited primary care prescribing capacity, and profound access inequities—with rural patients 37% less likely and Hispanic patients 24% less likely to receive treatment than metropolitan White counterparts. Closing this gap requires systematic implementation infrastructure, not expanded marketing.
Evidence for Implementation Readiness
Real-world effectiveness confirms pragmatic benefit
Large-scale pragmatic evidence now supports GLP-1 implementation beyond controlled trial populations. The SELECT trial (N=17,604) established semaglutide's cardiovascular benefit in patients with obesity without diabetes, demonstrating 9.4% mean body weight reduction and MACE reduction with HR 0.80 (95% CI: 0.72–0.90; NNT=67 over 40 months) (Lincoff et al., 2023). The VA Atlas study (N=2,191,223) mapped 175 health outcomes, confirming reduced risks across cardiometabolic, neurocognitive, and respiratory conditions with GLP-1 therapy, while identifying manageable risks including gastrointestinal events and drug-induced acute pancreatitis (HR 2.46) (Xie et al., 2025). Head-to-head real-world comparisons demonstrate no significant difference in cardiovascular outcomes between tirzepatide and semaglutide (HR 1.06; 95% CI: 0.95–1.18), supporting clinical equipoise for formulary decisions (Krüger et al., 2025). Tirzepatide achieves superior glycemic and weight outcomes (−10.2 kg vs. −6.1 kg at 12 months; P<0.001) in patients with diabetes (Hoog et al., 2025).
Health system implementation models demonstrate scalability
Multiple care models have achieved successful primary care integration. Pharmacist-led management at Community Care Physicians yielded 9.3% mean weight loss versus 5.1% with physician-only care (P=0.01), with $101,986 cost savings from inappropriate therapy deprescribing over 5 months (Crocetta et al., 2023). The University Hospitals Cleveland CINEMA program achieved 52% GLP-1 adoption among eligible high-cardiovascular-risk patients through multidisciplinary team-based care, with 81% of eligible patients initiated on evidence-based therapy within 3 months (Neeland et al., 2022). Michigan Medicine's Weight Navigation Program produced 12-lb mean weight loss (4.4% body weight) with 42% achieving ≥5% reduction through obesity specialist–PCP collaboration (Griauzde et al., 2024). Digital delivery shows promise: Second Nature's remote program achieved 19.1% weight loss among 12-month completers, though 60% withdrawal highlights engagement challenges (Richards et al., 2025).
Adherence and discontinuation require systematic support
Real-world persistence dramatically underperforms clinical trials. Only 42% of commercially insured patients persist beyond 12 weeks—the minimum duration for clinically meaningful benefit—with 30% discontinuing within 4 weeks before reaching target dose (Blue Health Intelligence, 2024). One-year persistence reaches only 32–47% depending on agent (Gleason et al., 2024). Discontinuation correlates with monthly copays >$60, higher social vulnerability index, younger age (18–34), and non-specialist prescribing. GI adverse events cause 10% discontinuation in trial settings, but real-world tolerance improves with proper titration support.
Equity gaps demand proactive intervention
Disparities in GLP-1 access are substantial and widening. Kim et al. (2025) documented that Hispanic patients are 24% less likely (OR 0.76; 95% CI: 0.75–0.76) and Asian patients 27% less likely (OR 0.73) than White patients to receive prescriptions. Patients in the highest social vulnerability quartile face 26% lower odds of treatment (OR 0.74; 95% CI: 0.74–0.75). Rural residents experience the steepest disparity at 37% reduced likelihood (OR 0.63). These gaps persist after adjustment and have not narrowed over time. Sarpatwari et al. (2025) found 37.2% fill rates for obesity-only prescriptions versus 64.6% for diabetes+obesity indications, reflecting insurance coverage differentials that disproportionately affect patients without diabetes.
Implementation Solution
Scalable GLP-1 program for primary care networks
Population: Adults with BMI ≥30 kg/m² (or ≥27 with weight-related comorbidity) in primary care network
Program Components:
1. Eligibility and Risk Stratification
EHR-based registry identifies eligible patients using automated BMI + comorbidity flags
Prioritization algorithm weights cardiovascular risk (CAC score, established ASCVD, HFrEF, CKD stages 2–4) per CINEMA criteria
Exclusion: personal/family history of medullary thyroid carcinoma, MEN2, pregnancy, active pancreatitis
2. Clinical Workflow and Team Roles
Phase | Timeline | Responsible Clinician | Key Activities |
|---|---|---|---|
Identification | Ongoing | Population Health Team | EHR registry query; risk stratification |
PCP Consult | Week 0 | Primary Care Physician | Eligibility confirmation; shared decision-making; cardiovascular risk assessment |
Insurance Navigation | Week 0–2 | Insurance Navigator/Medical Assistant | Prior authorization submission; manufacturer assistance enrollment; appeals if denied |
Medication Initiation | Week 2–4 | Clinical Pharmacist | Baseline labs; starting dose; injection teaching; adverse effect counseling |
Titration | Weeks 4–16 | Clinical Pharmacist/RN | Protocol-driven dose escalation per tolerance; GI symptom management; adherence assessment |
Maintenance Monitoring | Quarterly | PCP + Health Coach | Weight/cardiometabolic outcomes; behavioral support reinforcement; continuation criteria review |
Outcomes Tracking | Ongoing | Population Health Team | Registry updates; quality dashboard maintenance |
3. Coverage and Prior Authorization Pathway
Insurance verification at scheduling
Standardized PA template with BMI, comorbidities, failed lifestyle intervention documentation
Appeals protocol for initial denials (targeting 60%→85% approval rate)
Manufacturer savings card enrollment for commercial patients (target OOP <$50/month)
Financial toxicity screening with pathway to 340B pricing or patient assistance programs
4. EHR Registry and Population Health Infrastructure
Best Practice Alert for eligible patients without active prescription
Pharmacist clinical pathway order set with titration protocol
Outcomes dashboard: % eligible reached, % initiated, % at target dose, % achieving ≥5%/≥10% weight loss, adverse event rates, disparities monitoring by race/ethnicity/geography
5. Success Metrics
Process: ≥40% of eligible patients offered treatment; ≥70% PA approval; ≥60% 12-month persistence
Clinical: ≥50% achieving ≥5% weight loss; ≥25% achieving ≥10% weight loss; mean A1c reduction ≥0.8% (diabetic subset)
Equity: Prescription rates within 10% across race/ethnicity groups; rural uptake within 15% of urban
Figure 1: GLP-1 Implementation Pathway
Implementation Impact and Scalability
Population Reach Estimate: In a primary care network of 100,000 adults, approximately 42,000 meet BMI eligibility criteria; 15,000 have weight-related comorbidities warranting prioritization. At 40% program uptake, 6,000 patients would initiate GLP-1 therapy annually.
Expected Clinical Outcomes: Applying real-world effect sizes, 3,000 patients (50%) would achieve ≥5% weight loss; 1,500 (25%) would achieve ≥10%. Among the 2,400 with established cardiovascular disease, 36 MACE events would be prevented over 3 years (NNT=67).
Budget Impact Model:
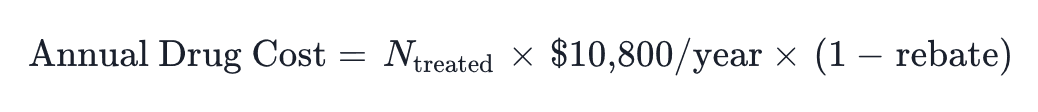
At net price of $6,500/patient/year (after 40% manufacturer rebate) for 6,000 patients: $39M annually. Cost-offset modeling incorporating reduced cardiovascular events, diabetes prevention, and bariatric surgery avoidance estimates $8–12M in downstream savings, yielding net incremental cost of ~$27–31M.
Equity Maintenance: Quarterly disparity audits with corrective action triggers; embedded insurance navigation; telehealth titration for rural access; FQHC partnership pathway.
Scalability: Model extends to Medicaid managed care (13 covering states) and Medicare (diabetes/CV indication) with modified coverage workflows. Rural adaptation via telehealth-first titration demonstrated feasible in VA TeleMOVE! and commercial digital programs.
References
Blue Health Intelligence. (2024). Real-world trends in GLP-1 treatment persistence and prescribing for weight management (Issue Brief). Blue Cross Blue Shield Association. https://www.bcbs.com/media/pdf/BHI_Issue_Brief_GLP1_Trends.pdf
Crocetta, N., Guay, K., & Watson, A. (2023). Outcomes and cost-effectiveness of a pharmacist-directed weight management service in a primary care setting. Family Practice, 40(2), 255–260. https://doi.org/10.1093/fampra/cmac110
Gleason, P. P., Urick, B. Y., Marshall, L. Z., & Friedman, N. J. (2024). Real-world persistence and adherence to glucagon-like peptide-1 receptor agonists among obese commercially insured adults without diabetes. Journal of Managed Care & Specialty Pharmacy, 30(8), 860–867. https://doi.org/10.18553/jmcp.2024.23332
Griauzde, D. H., Turner, C. D., Othman, A., Engelman, D., Glanville, J., Richardson, C. R., & Mizokami-Stout, K. (2024). Association of a weight navigation program with weight loss outcomes among primary care patients with obesity. JAMA Network Open, 7(5), e2412192. https://doi.org/10.1001/jamanetworkopen.2024.12192
Hoog, M. M., Vallarino, C., Maldonado, J. M., Garcia, E., Li, H., Buysman, E. K., & Grabner, M. (2025). Real-world effectiveness of tirzepatide versus semaglutide on HbA1c and weight in patients with type 2 diabetes. Diabetes Therapy, 16(11), 2237–2256. https://doi.org/10.1007/s13300-025-01794-9
Kim, C., Ross, J. S., Jastreboff, A. M., Roberts, E. T., Dhruva, S. S., & Zhang, Y. (2025). Uptake of and disparities in semaglutide and tirzepatide prescribing for obesity in the US. JAMA, 333(24), 2203–2206. https://doi.org/10.1001/jama.2025.4735
Krüger, N., Schneeweiss, S., Desai, R. J., Patorno, E., Glynn, R. J., Kulkarni, R. N., & Wexler, D. J. (2025). Cardiovascular outcomes of semaglutide and tirzepatide for patients with type 2 diabetes in clinical practice. Nature Medicine. Advance online publication. https://doi.org/10.1038/s41591-025-04102-x
Lincoff, A. M., Brown-Frandsen, K., Colhoun, H. M., Deanfield, J., Emerson, S. S., Esbjerg, S., Hardt-Lindberg, S., Hovingh, G. K., Kahn, S. E., Kushner, R. F., Lingvay, I., Oral, T. K., Michelsen, M. M., Plutzky, J., Tornøe, C. W., & Ryan, D. H. (2023). Semaglutide and cardiovascular outcomes in obesity without diabetes. New England Journal of Medicine, 389(24), 2221–2232. https://doi.org/10.1056/NEJMoa2307563
Neeland, I. J., Al-Kindi, S. G., Engelman, D., Campbell, M., Thomas, C., Gole, K., & Tang, W. H. W. (2022). Implementation of a cardiometabolic program with pharmacotherapy and team-based care in a high-risk population. Journal of the American Heart Association, 11(15), e024482. https://doi.org/10.1161/JAHA.120.024482
Williams, E., Rudowitz, R., & Bell, C. (2024, November 4). Medicaid coverage of and spending on GLP-1s. Kaiser Family Foundation. https://www.kff.org/medicaid/issue-brief/medicaid-coverage-of-and-spending-on-glp-1s/
Xie, Y., Choi, T., & Al-Aly, Z. (2025). Mapping the effectiveness and risks of GLP-1 receptor agonists. Nature Medicine, 31(3), 951–962. https://doi.org/10.1038/s41591-024-03412-w
The Implementation Gap
GLP-1 receptor agonists represent a landmark advance in obesity pharmacotherapy, yet a vast implementation chasm separates clinical efficacy from population-level impact. The SELECT trial demonstrated 20% cardiovascular risk reduction (HR 0.80; 95% CI: 0.72–0.90) in patients with obesity and established cardiovascular disease (Lincoff et al., 2023), while tirzepatide achieves 15–21% weight reduction in controlled settings. Despite this, only 2.3% of eligible patients receive GLP-1 prescriptions in real-world practice (Kim et al., 2025). Four structural barriers perpetuate this gap: prohibitive cost ($936–$1,349/month list price), restrictive payor policies (only 13 state Medicaid programs cover obesity indications), limited primary care prescribing capacity, and profound access inequities—with rural patients 37% less likely and Hispanic patients 24% less likely to receive treatment than metropolitan White counterparts. Closing this gap requires systematic implementation infrastructure, not expanded marketing.
Evidence for Implementation Readiness
Real-world effectiveness confirms pragmatic benefit
Large-scale pragmatic evidence now supports GLP-1 implementation beyond controlled trial populations. The SELECT trial (N=17,604) established semaglutide's cardiovascular benefit in patients with obesity without diabetes, demonstrating 9.4% mean body weight reduction and MACE reduction with HR 0.80 (95% CI: 0.72–0.90; NNT=67 over 40 months) (Lincoff et al., 2023). The VA Atlas study (N=2,191,223) mapped 175 health outcomes, confirming reduced risks across cardiometabolic, neurocognitive, and respiratory conditions with GLP-1 therapy, while identifying manageable risks including gastrointestinal events and drug-induced acute pancreatitis (HR 2.46) (Xie et al., 2025). Head-to-head real-world comparisons demonstrate no significant difference in cardiovascular outcomes between tirzepatide and semaglutide (HR 1.06; 95% CI: 0.95–1.18), supporting clinical equipoise for formulary decisions (Krüger et al., 2025). Tirzepatide achieves superior glycemic and weight outcomes (−10.2 kg vs. −6.1 kg at 12 months; P<0.001) in patients with diabetes (Hoog et al., 2025).
Health system implementation models demonstrate scalability
Multiple care models have achieved successful primary care integration. Pharmacist-led management at Community Care Physicians yielded 9.3% mean weight loss versus 5.1% with physician-only care (P=0.01), with $101,986 cost savings from inappropriate therapy deprescribing over 5 months (Crocetta et al., 2023). The University Hospitals Cleveland CINEMA program achieved 52% GLP-1 adoption among eligible high-cardiovascular-risk patients through multidisciplinary team-based care, with 81% of eligible patients initiated on evidence-based therapy within 3 months (Neeland et al., 2022). Michigan Medicine's Weight Navigation Program produced 12-lb mean weight loss (4.4% body weight) with 42% achieving ≥5% reduction through obesity specialist–PCP collaboration (Griauzde et al., 2024). Digital delivery shows promise: Second Nature's remote program achieved 19.1% weight loss among 12-month completers, though 60% withdrawal highlights engagement challenges (Richards et al., 2025).
Adherence and discontinuation require systematic support
Real-world persistence dramatically underperforms clinical trials. Only 42% of commercially insured patients persist beyond 12 weeks—the minimum duration for clinically meaningful benefit—with 30% discontinuing within 4 weeks before reaching target dose (Blue Health Intelligence, 2024). One-year persistence reaches only 32–47% depending on agent (Gleason et al., 2024). Discontinuation correlates with monthly copays >$60, higher social vulnerability index, younger age (18–34), and non-specialist prescribing. GI adverse events cause 10% discontinuation in trial settings, but real-world tolerance improves with proper titration support.
Equity gaps demand proactive intervention
Disparities in GLP-1 access are substantial and widening. Kim et al. (2025) documented that Hispanic patients are 24% less likely (OR 0.76; 95% CI: 0.75–0.76) and Asian patients 27% less likely (OR 0.73) than White patients to receive prescriptions. Patients in the highest social vulnerability quartile face 26% lower odds of treatment (OR 0.74; 95% CI: 0.74–0.75). Rural residents experience the steepest disparity at 37% reduced likelihood (OR 0.63). These gaps persist after adjustment and have not narrowed over time. Sarpatwari et al. (2025) found 37.2% fill rates for obesity-only prescriptions versus 64.6% for diabetes+obesity indications, reflecting insurance coverage differentials that disproportionately affect patients without diabetes.
Implementation Solution
Scalable GLP-1 program for primary care networks
Population: Adults with BMI ≥30 kg/m² (or ≥27 with weight-related comorbidity) in primary care network
Program Components:
1. Eligibility and Risk Stratification
EHR-based registry identifies eligible patients using automated BMI + comorbidity flags
Prioritization algorithm weights cardiovascular risk (CAC score, established ASCVD, HFrEF, CKD stages 2–4) per CINEMA criteria
Exclusion: personal/family history of medullary thyroid carcinoma, MEN2, pregnancy, active pancreatitis
2. Clinical Workflow and Team Roles
Phase | Timeline | Responsible Clinician | Key Activities |
|---|---|---|---|
Identification | Ongoing | Population Health Team | EHR registry query; risk stratification |
PCP Consult | Week 0 | Primary Care Physician | Eligibility confirmation; shared decision-making; cardiovascular risk assessment |
Insurance Navigation | Week 0–2 | Insurance Navigator/Medical Assistant | Prior authorization submission; manufacturer assistance enrollment; appeals if denied |
Medication Initiation | Week 2–4 | Clinical Pharmacist | Baseline labs; starting dose; injection teaching; adverse effect counseling |
Titration | Weeks 4–16 | Clinical Pharmacist/RN | Protocol-driven dose escalation per tolerance; GI symptom management; adherence assessment |
Maintenance Monitoring | Quarterly | PCP + Health Coach | Weight/cardiometabolic outcomes; behavioral support reinforcement; continuation criteria review |
Outcomes Tracking | Ongoing | Population Health Team | Registry updates; quality dashboard maintenance |
3. Coverage and Prior Authorization Pathway
Insurance verification at scheduling
Standardized PA template with BMI, comorbidities, failed lifestyle intervention documentation
Appeals protocol for initial denials (targeting 60%→85% approval rate)
Manufacturer savings card enrollment for commercial patients (target OOP <$50/month)
Financial toxicity screening with pathway to 340B pricing or patient assistance programs
4. EHR Registry and Population Health Infrastructure
Best Practice Alert for eligible patients without active prescription
Pharmacist clinical pathway order set with titration protocol
Outcomes dashboard: % eligible reached, % initiated, % at target dose, % achieving ≥5%/≥10% weight loss, adverse event rates, disparities monitoring by race/ethnicity/geography
5. Success Metrics
Process: ≥40% of eligible patients offered treatment; ≥70% PA approval; ≥60% 12-month persistence
Clinical: ≥50% achieving ≥5% weight loss; ≥25% achieving ≥10% weight loss; mean A1c reduction ≥0.8% (diabetic subset)
Equity: Prescription rates within 10% across race/ethnicity groups; rural uptake within 15% of urban
Figure 1: GLP-1 Implementation Pathway
Implementation Impact and Scalability
Population Reach Estimate: In a primary care network of 100,000 adults, approximately 42,000 meet BMI eligibility criteria; 15,000 have weight-related comorbidities warranting prioritization. At 40% program uptake, 6,000 patients would initiate GLP-1 therapy annually.
Expected Clinical Outcomes: Applying real-world effect sizes, 3,000 patients (50%) would achieve ≥5% weight loss; 1,500 (25%) would achieve ≥10%. Among the 2,400 with established cardiovascular disease, 36 MACE events would be prevented over 3 years (NNT=67).
Budget Impact Model:
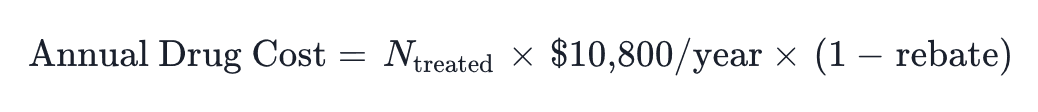
At net price of $6,500/patient/year (after 40% manufacturer rebate) for 6,000 patients: $39M annually. Cost-offset modeling incorporating reduced cardiovascular events, diabetes prevention, and bariatric surgery avoidance estimates $8–12M in downstream savings, yielding net incremental cost of ~$27–31M.
Equity Maintenance: Quarterly disparity audits with corrective action triggers; embedded insurance navigation; telehealth titration for rural access; FQHC partnership pathway.
Scalability: Model extends to Medicaid managed care (13 covering states) and Medicare (diabetes/CV indication) with modified coverage workflows. Rural adaptation via telehealth-first titration demonstrated feasible in VA TeleMOVE! and commercial digital programs.
References
Blue Health Intelligence. (2024). Real-world trends in GLP-1 treatment persistence and prescribing for weight management (Issue Brief). Blue Cross Blue Shield Association. https://www.bcbs.com/media/pdf/BHI_Issue_Brief_GLP1_Trends.pdf
Crocetta, N., Guay, K., & Watson, A. (2023). Outcomes and cost-effectiveness of a pharmacist-directed weight management service in a primary care setting. Family Practice, 40(2), 255–260. https://doi.org/10.1093/fampra/cmac110
Gleason, P. P., Urick, B. Y., Marshall, L. Z., & Friedman, N. J. (2024). Real-world persistence and adherence to glucagon-like peptide-1 receptor agonists among obese commercially insured adults without diabetes. Journal of Managed Care & Specialty Pharmacy, 30(8), 860–867. https://doi.org/10.18553/jmcp.2024.23332
Griauzde, D. H., Turner, C. D., Othman, A., Engelman, D., Glanville, J., Richardson, C. R., & Mizokami-Stout, K. (2024). Association of a weight navigation program with weight loss outcomes among primary care patients with obesity. JAMA Network Open, 7(5), e2412192. https://doi.org/10.1001/jamanetworkopen.2024.12192
Hoog, M. M., Vallarino, C., Maldonado, J. M., Garcia, E., Li, H., Buysman, E. K., & Grabner, M. (2025). Real-world effectiveness of tirzepatide versus semaglutide on HbA1c and weight in patients with type 2 diabetes. Diabetes Therapy, 16(11), 2237–2256. https://doi.org/10.1007/s13300-025-01794-9
Kim, C., Ross, J. S., Jastreboff, A. M., Roberts, E. T., Dhruva, S. S., & Zhang, Y. (2025). Uptake of and disparities in semaglutide and tirzepatide prescribing for obesity in the US. JAMA, 333(24), 2203–2206. https://doi.org/10.1001/jama.2025.4735
Krüger, N., Schneeweiss, S., Desai, R. J., Patorno, E., Glynn, R. J., Kulkarni, R. N., & Wexler, D. J. (2025). Cardiovascular outcomes of semaglutide and tirzepatide for patients with type 2 diabetes in clinical practice. Nature Medicine. Advance online publication. https://doi.org/10.1038/s41591-025-04102-x
Lincoff, A. M., Brown-Frandsen, K., Colhoun, H. M., Deanfield, J., Emerson, S. S., Esbjerg, S., Hardt-Lindberg, S., Hovingh, G. K., Kahn, S. E., Kushner, R. F., Lingvay, I., Oral, T. K., Michelsen, M. M., Plutzky, J., Tornøe, C. W., & Ryan, D. H. (2023). Semaglutide and cardiovascular outcomes in obesity without diabetes. New England Journal of Medicine, 389(24), 2221–2232. https://doi.org/10.1056/NEJMoa2307563
Neeland, I. J., Al-Kindi, S. G., Engelman, D., Campbell, M., Thomas, C., Gole, K., & Tang, W. H. W. (2022). Implementation of a cardiometabolic program with pharmacotherapy and team-based care in a high-risk population. Journal of the American Heart Association, 11(15), e024482. https://doi.org/10.1161/JAHA.120.024482
Williams, E., Rudowitz, R., & Bell, C. (2024, November 4). Medicaid coverage of and spending on GLP-1s. Kaiser Family Foundation. https://www.kff.org/medicaid/issue-brief/medicaid-coverage-of-and-spending-on-glp-1s/
Xie, Y., Choi, T., & Al-Aly, Z. (2025). Mapping the effectiveness and risks of GLP-1 receptor agonists. Nature Medicine, 31(3), 951–962. https://doi.org/10.1038/s41591-024-03412-w
The Implementation Gap
GLP-1 receptor agonists represent a landmark advance in obesity pharmacotherapy, yet a vast implementation chasm separates clinical efficacy from population-level impact. The SELECT trial demonstrated 20% cardiovascular risk reduction (HR 0.80; 95% CI: 0.72–0.90) in patients with obesity and established cardiovascular disease (Lincoff et al., 2023), while tirzepatide achieves 15–21% weight reduction in controlled settings. Despite this, only 2.3% of eligible patients receive GLP-1 prescriptions in real-world practice (Kim et al., 2025). Four structural barriers perpetuate this gap: prohibitive cost ($936–$1,349/month list price), restrictive payor policies (only 13 state Medicaid programs cover obesity indications), limited primary care prescribing capacity, and profound access inequities—with rural patients 37% less likely and Hispanic patients 24% less likely to receive treatment than metropolitan White counterparts. Closing this gap requires systematic implementation infrastructure, not expanded marketing.
Evidence for Implementation Readiness
Real-world effectiveness confirms pragmatic benefit
Large-scale pragmatic evidence now supports GLP-1 implementation beyond controlled trial populations. The SELECT trial (N=17,604) established semaglutide's cardiovascular benefit in patients with obesity without diabetes, demonstrating 9.4% mean body weight reduction and MACE reduction with HR 0.80 (95% CI: 0.72–0.90; NNT=67 over 40 months) (Lincoff et al., 2023). The VA Atlas study (N=2,191,223) mapped 175 health outcomes, confirming reduced risks across cardiometabolic, neurocognitive, and respiratory conditions with GLP-1 therapy, while identifying manageable risks including gastrointestinal events and drug-induced acute pancreatitis (HR 2.46) (Xie et al., 2025). Head-to-head real-world comparisons demonstrate no significant difference in cardiovascular outcomes between tirzepatide and semaglutide (HR 1.06; 95% CI: 0.95–1.18), supporting clinical equipoise for formulary decisions (Krüger et al., 2025). Tirzepatide achieves superior glycemic and weight outcomes (−10.2 kg vs. −6.1 kg at 12 months; P<0.001) in patients with diabetes (Hoog et al., 2025).
Health system implementation models demonstrate scalability
Multiple care models have achieved successful primary care integration. Pharmacist-led management at Community Care Physicians yielded 9.3% mean weight loss versus 5.1% with physician-only care (P=0.01), with $101,986 cost savings from inappropriate therapy deprescribing over 5 months (Crocetta et al., 2023). The University Hospitals Cleveland CINEMA program achieved 52% GLP-1 adoption among eligible high-cardiovascular-risk patients through multidisciplinary team-based care, with 81% of eligible patients initiated on evidence-based therapy within 3 months (Neeland et al., 2022). Michigan Medicine's Weight Navigation Program produced 12-lb mean weight loss (4.4% body weight) with 42% achieving ≥5% reduction through obesity specialist–PCP collaboration (Griauzde et al., 2024). Digital delivery shows promise: Second Nature's remote program achieved 19.1% weight loss among 12-month completers, though 60% withdrawal highlights engagement challenges (Richards et al., 2025).
Adherence and discontinuation require systematic support
Real-world persistence dramatically underperforms clinical trials. Only 42% of commercially insured patients persist beyond 12 weeks—the minimum duration for clinically meaningful benefit—with 30% discontinuing within 4 weeks before reaching target dose (Blue Health Intelligence, 2024). One-year persistence reaches only 32–47% depending on agent (Gleason et al., 2024). Discontinuation correlates with monthly copays >$60, higher social vulnerability index, younger age (18–34), and non-specialist prescribing. GI adverse events cause 10% discontinuation in trial settings, but real-world tolerance improves with proper titration support.
Equity gaps demand proactive intervention
Disparities in GLP-1 access are substantial and widening. Kim et al. (2025) documented that Hispanic patients are 24% less likely (OR 0.76; 95% CI: 0.75–0.76) and Asian patients 27% less likely (OR 0.73) than White patients to receive prescriptions. Patients in the highest social vulnerability quartile face 26% lower odds of treatment (OR 0.74; 95% CI: 0.74–0.75). Rural residents experience the steepest disparity at 37% reduced likelihood (OR 0.63). These gaps persist after adjustment and have not narrowed over time. Sarpatwari et al. (2025) found 37.2% fill rates for obesity-only prescriptions versus 64.6% for diabetes+obesity indications, reflecting insurance coverage differentials that disproportionately affect patients without diabetes.
Implementation Solution
Scalable GLP-1 program for primary care networks
Population: Adults with BMI ≥30 kg/m² (or ≥27 with weight-related comorbidity) in primary care network
Program Components:
1. Eligibility and Risk Stratification
EHR-based registry identifies eligible patients using automated BMI + comorbidity flags
Prioritization algorithm weights cardiovascular risk (CAC score, established ASCVD, HFrEF, CKD stages 2–4) per CINEMA criteria
Exclusion: personal/family history of medullary thyroid carcinoma, MEN2, pregnancy, active pancreatitis
2. Clinical Workflow and Team Roles
Phase | Timeline | Responsible Clinician | Key Activities |
|---|---|---|---|
Identification | Ongoing | Population Health Team | EHR registry query; risk stratification |
PCP Consult | Week 0 | Primary Care Physician | Eligibility confirmation; shared decision-making; cardiovascular risk assessment |
Insurance Navigation | Week 0–2 | Insurance Navigator/Medical Assistant | Prior authorization submission; manufacturer assistance enrollment; appeals if denied |
Medication Initiation | Week 2–4 | Clinical Pharmacist | Baseline labs; starting dose; injection teaching; adverse effect counseling |
Titration | Weeks 4–16 | Clinical Pharmacist/RN | Protocol-driven dose escalation per tolerance; GI symptom management; adherence assessment |
Maintenance Monitoring | Quarterly | PCP + Health Coach | Weight/cardiometabolic outcomes; behavioral support reinforcement; continuation criteria review |
Outcomes Tracking | Ongoing | Population Health Team | Registry updates; quality dashboard maintenance |
3. Coverage and Prior Authorization Pathway
Insurance verification at scheduling
Standardized PA template with BMI, comorbidities, failed lifestyle intervention documentation
Appeals protocol for initial denials (targeting 60%→85% approval rate)
Manufacturer savings card enrollment for commercial patients (target OOP <$50/month)
Financial toxicity screening with pathway to 340B pricing or patient assistance programs
4. EHR Registry and Population Health Infrastructure
Best Practice Alert for eligible patients without active prescription
Pharmacist clinical pathway order set with titration protocol
Outcomes dashboard: % eligible reached, % initiated, % at target dose, % achieving ≥5%/≥10% weight loss, adverse event rates, disparities monitoring by race/ethnicity/geography
5. Success Metrics
Process: ≥40% of eligible patients offered treatment; ≥70% PA approval; ≥60% 12-month persistence
Clinical: ≥50% achieving ≥5% weight loss; ≥25% achieving ≥10% weight loss; mean A1c reduction ≥0.8% (diabetic subset)
Equity: Prescription rates within 10% across race/ethnicity groups; rural uptake within 15% of urban
Figure 1: GLP-1 Implementation Pathway
Implementation Impact and Scalability
Population Reach Estimate: In a primary care network of 100,000 adults, approximately 42,000 meet BMI eligibility criteria; 15,000 have weight-related comorbidities warranting prioritization. At 40% program uptake, 6,000 patients would initiate GLP-1 therapy annually.
Expected Clinical Outcomes: Applying real-world effect sizes, 3,000 patients (50%) would achieve ≥5% weight loss; 1,500 (25%) would achieve ≥10%. Among the 2,400 with established cardiovascular disease, 36 MACE events would be prevented over 3 years (NNT=67).
Budget Impact Model:
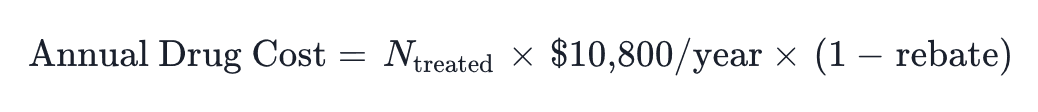
At net price of $6,500/patient/year (after 40% manufacturer rebate) for 6,000 patients: $39M annually. Cost-offset modeling incorporating reduced cardiovascular events, diabetes prevention, and bariatric surgery avoidance estimates $8–12M in downstream savings, yielding net incremental cost of ~$27–31M.
Equity Maintenance: Quarterly disparity audits with corrective action triggers; embedded insurance navigation; telehealth titration for rural access; FQHC partnership pathway.
Scalability: Model extends to Medicaid managed care (13 covering states) and Medicare (diabetes/CV indication) with modified coverage workflows. Rural adaptation via telehealth-first titration demonstrated feasible in VA TeleMOVE! and commercial digital programs.
References
Blue Health Intelligence. (2024). Real-world trends in GLP-1 treatment persistence and prescribing for weight management (Issue Brief). Blue Cross Blue Shield Association. https://www.bcbs.com/media/pdf/BHI_Issue_Brief_GLP1_Trends.pdf
Crocetta, N., Guay, K., & Watson, A. (2023). Outcomes and cost-effectiveness of a pharmacist-directed weight management service in a primary care setting. Family Practice, 40(2), 255–260. https://doi.org/10.1093/fampra/cmac110
Gleason, P. P., Urick, B. Y., Marshall, L. Z., & Friedman, N. J. (2024). Real-world persistence and adherence to glucagon-like peptide-1 receptor agonists among obese commercially insured adults without diabetes. Journal of Managed Care & Specialty Pharmacy, 30(8), 860–867. https://doi.org/10.18553/jmcp.2024.23332
Griauzde, D. H., Turner, C. D., Othman, A., Engelman, D., Glanville, J., Richardson, C. R., & Mizokami-Stout, K. (2024). Association of a weight navigation program with weight loss outcomes among primary care patients with obesity. JAMA Network Open, 7(5), e2412192. https://doi.org/10.1001/jamanetworkopen.2024.12192
Hoog, M. M., Vallarino, C., Maldonado, J. M., Garcia, E., Li, H., Buysman, E. K., & Grabner, M. (2025). Real-world effectiveness of tirzepatide versus semaglutide on HbA1c and weight in patients with type 2 diabetes. Diabetes Therapy, 16(11), 2237–2256. https://doi.org/10.1007/s13300-025-01794-9
Kim, C., Ross, J. S., Jastreboff, A. M., Roberts, E. T., Dhruva, S. S., & Zhang, Y. (2025). Uptake of and disparities in semaglutide and tirzepatide prescribing for obesity in the US. JAMA, 333(24), 2203–2206. https://doi.org/10.1001/jama.2025.4735
Krüger, N., Schneeweiss, S., Desai, R. J., Patorno, E., Glynn, R. J., Kulkarni, R. N., & Wexler, D. J. (2025). Cardiovascular outcomes of semaglutide and tirzepatide for patients with type 2 diabetes in clinical practice. Nature Medicine. Advance online publication. https://doi.org/10.1038/s41591-025-04102-x
Lincoff, A. M., Brown-Frandsen, K., Colhoun, H. M., Deanfield, J., Emerson, S. S., Esbjerg, S., Hardt-Lindberg, S., Hovingh, G. K., Kahn, S. E., Kushner, R. F., Lingvay, I., Oral, T. K., Michelsen, M. M., Plutzky, J., Tornøe, C. W., & Ryan, D. H. (2023). Semaglutide and cardiovascular outcomes in obesity without diabetes. New England Journal of Medicine, 389(24), 2221–2232. https://doi.org/10.1056/NEJMoa2307563
Neeland, I. J., Al-Kindi, S. G., Engelman, D., Campbell, M., Thomas, C., Gole, K., & Tang, W. H. W. (2022). Implementation of a cardiometabolic program with pharmacotherapy and team-based care in a high-risk population. Journal of the American Heart Association, 11(15), e024482. https://doi.org/10.1161/JAHA.120.024482
Williams, E., Rudowitz, R., & Bell, C. (2024, November 4). Medicaid coverage of and spending on GLP-1s. Kaiser Family Foundation. https://www.kff.org/medicaid/issue-brief/medicaid-coverage-of-and-spending-on-glp-1s/
Xie, Y., Choi, T., & Al-Aly, Z. (2025). Mapping the effectiveness and risks of GLP-1 receptor agonists. Nature Medicine, 31(3), 951–962. https://doi.org/10.1038/s41591-024-03412-w
Turn evidence into everyday care.
No spam, unsubscribe anytime.