Rapid Diagnostic Testing for Tuberculosis in Primary Care Settings
Verified by Sahaj Satani from ImplementMD



The Implementation Gap
Tuberculosis kills approximately 1.25 million people annually, with diagnostic delay remaining a critical driver of mortality and transmission (WHO Global TB Report, 2024). Despite WHO endorsement of Xpert MTB/RIF in 2010 and subsequent rapid molecular diagnostics, only 48% of newly diagnosed TB patients globally received initial testing with a WHO-recommended rapid diagnostic test in 2023—far below the 100% target set for 2027. A mere 35% of TB diagnostic sites in high-burden countries have access to these tests, with South-East Asia reporting the lowest regional coverage at 39% (WHO, 2024). Critical barriers include infrastructure limitations, unclear clinical workflows, inadequate training systems, and financing gaps—particularly for decentralization away from centralized reference laboratories to primary care facilities where patients first present.
Evidence for Implementation Readiness
Diagnostic Performance in Real-World Primary Care Settings
Multi-country diagnostic accuracy studies provide robust performance data for primary care implementation. The landmark Dorman et al. (2018) prospective study across 8 countries (n=2,368) demonstrated that Xpert MTB/RIF Ultra achieves 88% sensitivity (95% CI: 85-91%) and 96% specificity (95% CI: 94-97%) against culture reference in routine settings. Critically, Ultra shows superior performance in challenging populations: 63% sensitivity (vs. 46% for original Xpert) in smear-negative cases and 90% sensitivity (vs. 77%) in HIV-positive patients. For decentralized settings, the Penn-Nicholson et al. (2021) WHO evaluation of Truenat across 19 primary healthcare centres in Peru, India, Ethiopia, and Papua New Guinea (n=1,356) demonstrated 80% sensitivity (95% CI: 75-84%) and 96% specificity (95% CI: 95-97%) for Truenat MTB Plus—a platform specifically designed for peripheral facilities with battery operation and tolerance up to 40°C.
For HIV-TB co-infection—responsible for significant mortality in high-burden regions—lateral flow lipoarabinomannan (LF-LAM) testing offers true point-of-care capability. The Broger et al. (2020) individual participant meta-analysis (n=1,595 HIV-positive adults) demonstrated that SILVAMP-LAM (FujiLAM) achieves 87.1% sensitivity (95% CI: 79.3-93.6%) in patients with CD4 ≤100 cells/μL, compared to only 56% for AlereLAM. When combined with sputum Xpert, 92% of all TB cases are detected in HIV-positive populations.
Implementation Evidence from Health Systems
India's RNTCP Demonstration Study (Sachdeva et al., 2015) tested 70,556 presumptive TB patients across 18 sub-district tuberculosis units, achieving a 39% increase in case notification (aIRR 1.39; 95% CI: 1.18-1.64) and 5-fold increase in rifampicin-resistant TB detection following Xpert decentralization to microscopy center level. Operational feasibility was high, with 99.1% valid results.
South Africa's national experience provides perhaps the most comprehensive implementation evidence. The Khayelitsha decentralization program (Cox et al., 2015) documented dramatic reductions in time-to-treatment: from 71 days (pre-program) to 28 days (decentralized care) to just 8 days (post-Xpert). The nationwide retrospective cohort (Cox et al., 2017; n=5,036) showed treatment initiation within 6 months improved from 55% to 63%, and pre-treatment mortality fell from 17.5% to 5.8% following Xpert implementation. The POC-based Lessells et al. (2017) cluster-randomized trial demonstrated point-of-care Xpert reduces median time-to-treatment to 1 day versus 7 days for laboratory-based testing.
Tanzania's MDR-TB program showed Xpert-based diagnosis reduced time-to-treatment from 155 days (phenotypic DST era) to 26 days (Xpert era) for drug-resistant cases.
Cost-Effectiveness and Financing
Economic evaluations consistently demonstrate cost-effectiveness at current pricing. In Mozambique, Orlando et al. (2018) calculated an incremental cost-effectiveness ratio of $56.54 per DALY averted for Xpert versus standard care in HIV-positive patients—well below the GDP-per-capita threshold. The Kenya LF-LAM analysis (Yakhelef et al., 2020) found even more favorable ratios: €4.6 (~$5) per DALY averted for LF-LAM-based algorithms in HIV-positive patients with CD4 <200.
Current per-test costs through Global Drug Facility negotiated pricing: Xpert MTB/RIF Ultra at $7.97, Truenat MTB Plus at $7.90, and AlereLAM at ~$3.50. Full operational costs including maintenance surcharges total approximately $20-26 per Xpert test at volumes of ≥3 tests daily.
Equity Impacts
Rapid diagnostics disproportionately benefit populations facing the greatest diagnostic delays. Only 7 of 30 high-burden countries report >50% of diagnostic sites with rapid test access (WHO, 2024). Rural populations experience 22-78% higher odds of diagnostic delay compared to urban populations. Pediatric TB presents unique challenges—78.9% of children under 5 years present with extrapulmonary TB, which has lower diagnostic yields. The systematic review by Naidoo et al. (2022) found molecular diagnostics reduce diagnostic delay by 40 days for drug-resistant TB compared to culture-based approaches.
Implementation Solution
Scalable Primary Care TB Diagnostic Program Framework
Site Selection Criteria: Prioritize facilities meeting ≥3 criteria: (1) TB case notification >50 annually; (2) HIV prevalence >5%; (3) distance >30 km from reference laboratory; (4) existing microscopy services; (5) stable electricity (or solar backup feasibility).
Test Selection Algorithm:
First-line for all presumptive TB: Xpert MTB/RIF Ultra (preferred) or Truenat MTB Plus (where infrastructure constraints exist)
HIV-positive patients with CD4 <200 or serious illness: Add urine LF-LAM (FujiLAM when available; AlereLAM currently)
Pediatric patients: Stool-based Xpert when respiratory specimens unavailable
Clinical Workflow Integration:
Day | Activity | Responsible Personnel | Output |
|---|---|---|---|
Day 0 | Patient presents with respiratory symptoms; clinician applies screening criteria (cough ≥2 weeks, fever, weight loss, night sweats, HIV status) | Clinical officer/nurse | Presumptive TB identification |
Day 0 | Sputum collection (spot specimen); urine collection if HIV+ | Nurse/CHW | Specimens for testing |
Day 0-1 | Rapid molecular test performed | Trained operator (lab tech/nurse) | Xpert/Truenat result |
Day 0-1 | Result recorded in TB register; clinician notified | Laboratory/data clerk | Documentation complete |
Day 1 | Positive result: Patient counseling, chest X-ray, treatment initiation | Clinical officer | TB treatment started |
Day 1-7 | Negative result with high clinical suspicion: Repeat testing, clinical diagnosis consideration | Clinical officer | Clinical pathway determination |
Day 7 | Rifampicin-resistant: Referral to DR-TB treatment site | Program coordinator | DR-TB care linkage |
Day 14-30 | Treatment adherence monitoring; contact tracing initiated | CHW/nurse | Treatment cascade support |
Staffing and Training Model:
Operators: 2 trained staff per site (primary + backup); 2-3 day competency-based training
Competency standards: ≥90% practical assessment, ≥80% written examination
Task-shifting: Evidence supports nurse and community health worker operation with equivalent outcomes to laboratory technicians (WHO Implementation Manual)
Quality Assurance Framework:
Monthly indicator monitoring: error rates (target <5%), invalid rates, turnaround time
Quarterly dried culture spot (DCS) proficiency testing panels via national TB reference laboratory
Annual module calibration ($450/4 modules) with service contract
Super-user network for basic troubleshooting
Data Systems:
Paper-based TB laboratory registers with digital backup
GxAlert or equivalent connectivity solution for automatic result transmission
Integration with national TB surveillance (e.g., DHIS2)
Dashboard for key performance indicators
Financing Pathway:
Year 1-2: Global Fund/PEPFAR catalytic funding for equipment and initial cartridge procurement via GDF
Year 2-3: Government budget line allocation for consumables (cartridges, maintenance)
Year 3+: Domestic financing with donor support for quality assurance and scale-up
Cost recovery not recommended given public health priority
Figure 1: Primary Care TB Diagnostic Pathway
Implementation Impact & Scalability
Projected Impact for a District of 500,000 Population
TB Case Detection (High-Burden Setting):
Using WHO incidence estimates and implementation study yields:
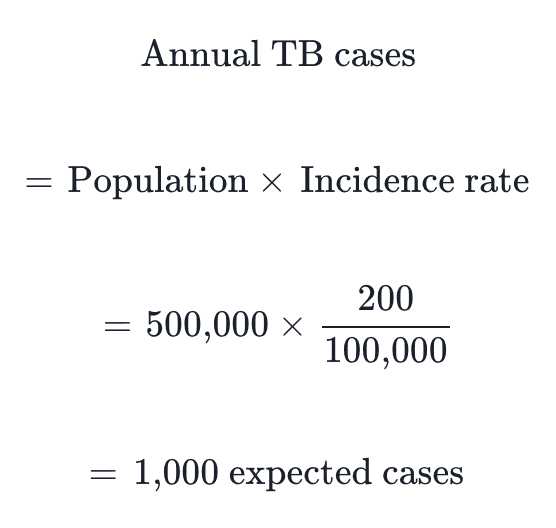
Metric | Conventional (Smear) | Rapid Molecular | Source |
|---|---|---|---|
Annual cases detected | 600-700 | 850-900 | Sachdeva et al., 2015 |
Bacteriological confirmation | 60% | 85% | India RNTCP data |
RR-TB detected | 15-20 | 75-100 | 5-fold increase documented |
Median diagnostic delay | 34 days | 2 days | Cox et al., 2015 |
Resource Requirements:
Sites requiring rapid diagnostics: 8-12 primary care facilities (based on TB burden distribution)
Health workers to train: 16-24 (2 per site); 2-3 days training each
Equipment cost: $140,000-228,000 (8-12 GeneXpert 4-module instruments at $17,500-19,000)
Annual cartridge cost: $160,000-180,000 (20,000 tests × $8)
Total Year 1 budget: $350,000-450,000; Years 2+: $180,000-220,000
Equity Impact: Rural TB detection rates projected to increase from 35% to 75% of expected cases with decentralization (WHO, 2024). Time-to-treatment for rifampicin-resistant TB reduced from 44 days to 8 days based on South African evidence (Cox et al., 2017).
Scalability: This model has been validated across India (70,556 patients), South Africa (>100,000 tests annually), and multiple East African settings. Key success factors include supply chain reliability, quality assurance systems, and treatment linkage protocols.
Evidence Gaps: Prospective trials comparing mortality outcomes between rapid molecular versus microscopy-based algorithms in primary care remain limited—the XTEND trial (Churchyard et al., 2015) found no mortality benefit despite improved diagnosis, highlighting the need for health systems strengthening beyond diagnostics alone.
References
Dorman SE, Schumacher SG, Alland D, et al. Xpert MTB/RIF Ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre diagnostic accuracy study. Lancet Infect Dis. 2018;18(1):76-84. https://doi.org/10.1016/S1473-3099(17)30691-6
Penn-Nicholson A, Gomathi SN, Engel N, et al. A prospective multicentre diagnostic accuracy study for the Truenat tuberculosis assays. Eur Respir J. 2021;58(5):2100526. https://doi.org/10.1183/13993003.00526-2021
Broger T, Nicol MP, Székely R, et al. Diagnostic accuracy of a novel tuberculosis point-of-care urine lipoarabinomannan assay for people living with HIV: A meta-analysis of individual in- and outpatient data. PLoS Med. 2020;17(5):e1003113. https://doi.org/10.1371/journal.pmed.1003113
Sachdeva KS, Raizada N, Sreenivas A, et al. Use of Xpert MTB/RIF in decentralized public health settings and its effect on pulmonary TB and DR-TB case finding in India. PLoS One. 2015;10(5):e0126065. https://doi.org/10.1371/journal.pone.0126065
Cox HS, Daniels JF, Muller O, et al. Impact of decentralized care and the Xpert MTB/RIF test on rifampicin-resistant tuberculosis treatment initiation in Khayelitsha, South Africa. Open Forum Infect Dis. 2015;2(1):ofv014. https://doi.org/10.1093/ofid/ofv014
Cox H, Dickson-Hall L, Ndjeka N, et al. Delays and loss to follow-up before treatment of drug-resistant tuberculosis following implementation of Xpert MTB/RIF in South Africa: a retrospective cohort study. PLoS Med. 2017;14(2):e1002238. https://doi.org/10.1371/journal.pmed.1002238
Churchyard GJ, Stevens WS, Mametja LD, et al. Xpert MTB/RIF versus sputum microscopy as the initial diagnostic test for tuberculosis: a cluster-randomised trial embedded in South African roll-out of Xpert MTB/RIF. Lancet Glob Health. 2015;3(8):e450-e457. https://doi.org/10.1016/S2214-109X(15)00100-X
Lessells RJ, Cooke GS, McGrath N, et al. Impact of point-of-care Xpert MTB/RIF on tuberculosis treatment initiation: a cluster-randomized trial. Am J Respir Crit Care Med. 2017;196(7):901-910. https://doi.org/10.1164/rccm.201702-0278OC
Orlando S, Triulzi I, Ciccacci F, et al. Delayed diagnosis and treatment of tuberculosis in HIV+ patients in Mozambique: a cost-effectiveness analysis of screening protocols based on four symptom screening, smear microscopy, urine LAM test and Xpert MTB/RIF. PLoS One. 2018;13(7):e0200523. https://doi.org/10.1371/journal.pone.0200523
Yakhelef N, Audibert M, Ferlazzo G, et al. Cost-effectiveness of diagnostic algorithms including lateral-flow urine lipoarabinomannan for HIV-positive patients with symptoms of tuberculosis in Kenya. PLoS One. 2020;15(1):e0227138. https://doi.org/10.1371/journal.pone.0227138
World Health Organization. Global Tuberculosis Report 2024. Geneva: WHO; 2024. https://www.who.int/teams/global-programme-on-tuberculosis-and-lung-health/tb-reports
Bjerrum S, Schiller I, Dendukuri N, et al. Lateral flow urine lipoarabinomannan assay for detecting active tuberculosis in people living with HIV. Cochrane Database Syst Rev. 2019;10(10):CD011420. https://doi.org/10.1002/14651858.CD011420.pub3
The Implementation Gap
Tuberculosis kills approximately 1.25 million people annually, with diagnostic delay remaining a critical driver of mortality and transmission (WHO Global TB Report, 2024). Despite WHO endorsement of Xpert MTB/RIF in 2010 and subsequent rapid molecular diagnostics, only 48% of newly diagnosed TB patients globally received initial testing with a WHO-recommended rapid diagnostic test in 2023—far below the 100% target set for 2027. A mere 35% of TB diagnostic sites in high-burden countries have access to these tests, with South-East Asia reporting the lowest regional coverage at 39% (WHO, 2024). Critical barriers include infrastructure limitations, unclear clinical workflows, inadequate training systems, and financing gaps—particularly for decentralization away from centralized reference laboratories to primary care facilities where patients first present.
Evidence for Implementation Readiness
Diagnostic Performance in Real-World Primary Care Settings
Multi-country diagnostic accuracy studies provide robust performance data for primary care implementation. The landmark Dorman et al. (2018) prospective study across 8 countries (n=2,368) demonstrated that Xpert MTB/RIF Ultra achieves 88% sensitivity (95% CI: 85-91%) and 96% specificity (95% CI: 94-97%) against culture reference in routine settings. Critically, Ultra shows superior performance in challenging populations: 63% sensitivity (vs. 46% for original Xpert) in smear-negative cases and 90% sensitivity (vs. 77%) in HIV-positive patients. For decentralized settings, the Penn-Nicholson et al. (2021) WHO evaluation of Truenat across 19 primary healthcare centres in Peru, India, Ethiopia, and Papua New Guinea (n=1,356) demonstrated 80% sensitivity (95% CI: 75-84%) and 96% specificity (95% CI: 95-97%) for Truenat MTB Plus—a platform specifically designed for peripheral facilities with battery operation and tolerance up to 40°C.
For HIV-TB co-infection—responsible for significant mortality in high-burden regions—lateral flow lipoarabinomannan (LF-LAM) testing offers true point-of-care capability. The Broger et al. (2020) individual participant meta-analysis (n=1,595 HIV-positive adults) demonstrated that SILVAMP-LAM (FujiLAM) achieves 87.1% sensitivity (95% CI: 79.3-93.6%) in patients with CD4 ≤100 cells/μL, compared to only 56% for AlereLAM. When combined with sputum Xpert, 92% of all TB cases are detected in HIV-positive populations.
Implementation Evidence from Health Systems
India's RNTCP Demonstration Study (Sachdeva et al., 2015) tested 70,556 presumptive TB patients across 18 sub-district tuberculosis units, achieving a 39% increase in case notification (aIRR 1.39; 95% CI: 1.18-1.64) and 5-fold increase in rifampicin-resistant TB detection following Xpert decentralization to microscopy center level. Operational feasibility was high, with 99.1% valid results.
South Africa's national experience provides perhaps the most comprehensive implementation evidence. The Khayelitsha decentralization program (Cox et al., 2015) documented dramatic reductions in time-to-treatment: from 71 days (pre-program) to 28 days (decentralized care) to just 8 days (post-Xpert). The nationwide retrospective cohort (Cox et al., 2017; n=5,036) showed treatment initiation within 6 months improved from 55% to 63%, and pre-treatment mortality fell from 17.5% to 5.8% following Xpert implementation. The POC-based Lessells et al. (2017) cluster-randomized trial demonstrated point-of-care Xpert reduces median time-to-treatment to 1 day versus 7 days for laboratory-based testing.
Tanzania's MDR-TB program showed Xpert-based diagnosis reduced time-to-treatment from 155 days (phenotypic DST era) to 26 days (Xpert era) for drug-resistant cases.
Cost-Effectiveness and Financing
Economic evaluations consistently demonstrate cost-effectiveness at current pricing. In Mozambique, Orlando et al. (2018) calculated an incremental cost-effectiveness ratio of $56.54 per DALY averted for Xpert versus standard care in HIV-positive patients—well below the GDP-per-capita threshold. The Kenya LF-LAM analysis (Yakhelef et al., 2020) found even more favorable ratios: €4.6 (~$5) per DALY averted for LF-LAM-based algorithms in HIV-positive patients with CD4 <200.
Current per-test costs through Global Drug Facility negotiated pricing: Xpert MTB/RIF Ultra at $7.97, Truenat MTB Plus at $7.90, and AlereLAM at ~$3.50. Full operational costs including maintenance surcharges total approximately $20-26 per Xpert test at volumes of ≥3 tests daily.
Equity Impacts
Rapid diagnostics disproportionately benefit populations facing the greatest diagnostic delays. Only 7 of 30 high-burden countries report >50% of diagnostic sites with rapid test access (WHO, 2024). Rural populations experience 22-78% higher odds of diagnostic delay compared to urban populations. Pediatric TB presents unique challenges—78.9% of children under 5 years present with extrapulmonary TB, which has lower diagnostic yields. The systematic review by Naidoo et al. (2022) found molecular diagnostics reduce diagnostic delay by 40 days for drug-resistant TB compared to culture-based approaches.
Implementation Solution
Scalable Primary Care TB Diagnostic Program Framework
Site Selection Criteria: Prioritize facilities meeting ≥3 criteria: (1) TB case notification >50 annually; (2) HIV prevalence >5%; (3) distance >30 km from reference laboratory; (4) existing microscopy services; (5) stable electricity (or solar backup feasibility).
Test Selection Algorithm:
First-line for all presumptive TB: Xpert MTB/RIF Ultra (preferred) or Truenat MTB Plus (where infrastructure constraints exist)
HIV-positive patients with CD4 <200 or serious illness: Add urine LF-LAM (FujiLAM when available; AlereLAM currently)
Pediatric patients: Stool-based Xpert when respiratory specimens unavailable
Clinical Workflow Integration:
Day | Activity | Responsible Personnel | Output |
|---|---|---|---|
Day 0 | Patient presents with respiratory symptoms; clinician applies screening criteria (cough ≥2 weeks, fever, weight loss, night sweats, HIV status) | Clinical officer/nurse | Presumptive TB identification |
Day 0 | Sputum collection (spot specimen); urine collection if HIV+ | Nurse/CHW | Specimens for testing |
Day 0-1 | Rapid molecular test performed | Trained operator (lab tech/nurse) | Xpert/Truenat result |
Day 0-1 | Result recorded in TB register; clinician notified | Laboratory/data clerk | Documentation complete |
Day 1 | Positive result: Patient counseling, chest X-ray, treatment initiation | Clinical officer | TB treatment started |
Day 1-7 | Negative result with high clinical suspicion: Repeat testing, clinical diagnosis consideration | Clinical officer | Clinical pathway determination |
Day 7 | Rifampicin-resistant: Referral to DR-TB treatment site | Program coordinator | DR-TB care linkage |
Day 14-30 | Treatment adherence monitoring; contact tracing initiated | CHW/nurse | Treatment cascade support |
Staffing and Training Model:
Operators: 2 trained staff per site (primary + backup); 2-3 day competency-based training
Competency standards: ≥90% practical assessment, ≥80% written examination
Task-shifting: Evidence supports nurse and community health worker operation with equivalent outcomes to laboratory technicians (WHO Implementation Manual)
Quality Assurance Framework:
Monthly indicator monitoring: error rates (target <5%), invalid rates, turnaround time
Quarterly dried culture spot (DCS) proficiency testing panels via national TB reference laboratory
Annual module calibration ($450/4 modules) with service contract
Super-user network for basic troubleshooting
Data Systems:
Paper-based TB laboratory registers with digital backup
GxAlert or equivalent connectivity solution for automatic result transmission
Integration with national TB surveillance (e.g., DHIS2)
Dashboard for key performance indicators
Financing Pathway:
Year 1-2: Global Fund/PEPFAR catalytic funding for equipment and initial cartridge procurement via GDF
Year 2-3: Government budget line allocation for consumables (cartridges, maintenance)
Year 3+: Domestic financing with donor support for quality assurance and scale-up
Cost recovery not recommended given public health priority
Figure 1: Primary Care TB Diagnostic Pathway
Implementation Impact & Scalability
Projected Impact for a District of 500,000 Population
TB Case Detection (High-Burden Setting):
Using WHO incidence estimates and implementation study yields:

Metric | Conventional (Smear) | Rapid Molecular | Source |
|---|---|---|---|
Annual cases detected | 600-700 | 850-900 | Sachdeva et al., 2015 |
Bacteriological confirmation | 60% | 85% | India RNTCP data |
RR-TB detected | 15-20 | 75-100 | 5-fold increase documented |
Median diagnostic delay | 34 days | 2 days | Cox et al., 2015 |
Resource Requirements:
Sites requiring rapid diagnostics: 8-12 primary care facilities (based on TB burden distribution)
Health workers to train: 16-24 (2 per site); 2-3 days training each
Equipment cost: $140,000-228,000 (8-12 GeneXpert 4-module instruments at $17,500-19,000)
Annual cartridge cost: $160,000-180,000 (20,000 tests × $8)
Total Year 1 budget: $350,000-450,000; Years 2+: $180,000-220,000
Equity Impact: Rural TB detection rates projected to increase from 35% to 75% of expected cases with decentralization (WHO, 2024). Time-to-treatment for rifampicin-resistant TB reduced from 44 days to 8 days based on South African evidence (Cox et al., 2017).
Scalability: This model has been validated across India (70,556 patients), South Africa (>100,000 tests annually), and multiple East African settings. Key success factors include supply chain reliability, quality assurance systems, and treatment linkage protocols.
Evidence Gaps: Prospective trials comparing mortality outcomes between rapid molecular versus microscopy-based algorithms in primary care remain limited—the XTEND trial (Churchyard et al., 2015) found no mortality benefit despite improved diagnosis, highlighting the need for health systems strengthening beyond diagnostics alone.
References
Dorman SE, Schumacher SG, Alland D, et al. Xpert MTB/RIF Ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre diagnostic accuracy study. Lancet Infect Dis. 2018;18(1):76-84. https://doi.org/10.1016/S1473-3099(17)30691-6
Penn-Nicholson A, Gomathi SN, Engel N, et al. A prospective multicentre diagnostic accuracy study for the Truenat tuberculosis assays. Eur Respir J. 2021;58(5):2100526. https://doi.org/10.1183/13993003.00526-2021
Broger T, Nicol MP, Székely R, et al. Diagnostic accuracy of a novel tuberculosis point-of-care urine lipoarabinomannan assay for people living with HIV: A meta-analysis of individual in- and outpatient data. PLoS Med. 2020;17(5):e1003113. https://doi.org/10.1371/journal.pmed.1003113
Sachdeva KS, Raizada N, Sreenivas A, et al. Use of Xpert MTB/RIF in decentralized public health settings and its effect on pulmonary TB and DR-TB case finding in India. PLoS One. 2015;10(5):e0126065. https://doi.org/10.1371/journal.pone.0126065
Cox HS, Daniels JF, Muller O, et al. Impact of decentralized care and the Xpert MTB/RIF test on rifampicin-resistant tuberculosis treatment initiation in Khayelitsha, South Africa. Open Forum Infect Dis. 2015;2(1):ofv014. https://doi.org/10.1093/ofid/ofv014
Cox H, Dickson-Hall L, Ndjeka N, et al. Delays and loss to follow-up before treatment of drug-resistant tuberculosis following implementation of Xpert MTB/RIF in South Africa: a retrospective cohort study. PLoS Med. 2017;14(2):e1002238. https://doi.org/10.1371/journal.pmed.1002238
Churchyard GJ, Stevens WS, Mametja LD, et al. Xpert MTB/RIF versus sputum microscopy as the initial diagnostic test for tuberculosis: a cluster-randomised trial embedded in South African roll-out of Xpert MTB/RIF. Lancet Glob Health. 2015;3(8):e450-e457. https://doi.org/10.1016/S2214-109X(15)00100-X
Lessells RJ, Cooke GS, McGrath N, et al. Impact of point-of-care Xpert MTB/RIF on tuberculosis treatment initiation: a cluster-randomized trial. Am J Respir Crit Care Med. 2017;196(7):901-910. https://doi.org/10.1164/rccm.201702-0278OC
Orlando S, Triulzi I, Ciccacci F, et al. Delayed diagnosis and treatment of tuberculosis in HIV+ patients in Mozambique: a cost-effectiveness analysis of screening protocols based on four symptom screening, smear microscopy, urine LAM test and Xpert MTB/RIF. PLoS One. 2018;13(7):e0200523. https://doi.org/10.1371/journal.pone.0200523
Yakhelef N, Audibert M, Ferlazzo G, et al. Cost-effectiveness of diagnostic algorithms including lateral-flow urine lipoarabinomannan for HIV-positive patients with symptoms of tuberculosis in Kenya. PLoS One. 2020;15(1):e0227138. https://doi.org/10.1371/journal.pone.0227138
World Health Organization. Global Tuberculosis Report 2024. Geneva: WHO; 2024. https://www.who.int/teams/global-programme-on-tuberculosis-and-lung-health/tb-reports
Bjerrum S, Schiller I, Dendukuri N, et al. Lateral flow urine lipoarabinomannan assay for detecting active tuberculosis in people living with HIV. Cochrane Database Syst Rev. 2019;10(10):CD011420. https://doi.org/10.1002/14651858.CD011420.pub3
The Implementation Gap
Tuberculosis kills approximately 1.25 million people annually, with diagnostic delay remaining a critical driver of mortality and transmission (WHO Global TB Report, 2024). Despite WHO endorsement of Xpert MTB/RIF in 2010 and subsequent rapid molecular diagnostics, only 48% of newly diagnosed TB patients globally received initial testing with a WHO-recommended rapid diagnostic test in 2023—far below the 100% target set for 2027. A mere 35% of TB diagnostic sites in high-burden countries have access to these tests, with South-East Asia reporting the lowest regional coverage at 39% (WHO, 2024). Critical barriers include infrastructure limitations, unclear clinical workflows, inadequate training systems, and financing gaps—particularly for decentralization away from centralized reference laboratories to primary care facilities where patients first present.
Evidence for Implementation Readiness
Diagnostic Performance in Real-World Primary Care Settings
Multi-country diagnostic accuracy studies provide robust performance data for primary care implementation. The landmark Dorman et al. (2018) prospective study across 8 countries (n=2,368) demonstrated that Xpert MTB/RIF Ultra achieves 88% sensitivity (95% CI: 85-91%) and 96% specificity (95% CI: 94-97%) against culture reference in routine settings. Critically, Ultra shows superior performance in challenging populations: 63% sensitivity (vs. 46% for original Xpert) in smear-negative cases and 90% sensitivity (vs. 77%) in HIV-positive patients. For decentralized settings, the Penn-Nicholson et al. (2021) WHO evaluation of Truenat across 19 primary healthcare centres in Peru, India, Ethiopia, and Papua New Guinea (n=1,356) demonstrated 80% sensitivity (95% CI: 75-84%) and 96% specificity (95% CI: 95-97%) for Truenat MTB Plus—a platform specifically designed for peripheral facilities with battery operation and tolerance up to 40°C.
For HIV-TB co-infection—responsible for significant mortality in high-burden regions—lateral flow lipoarabinomannan (LF-LAM) testing offers true point-of-care capability. The Broger et al. (2020) individual participant meta-analysis (n=1,595 HIV-positive adults) demonstrated that SILVAMP-LAM (FujiLAM) achieves 87.1% sensitivity (95% CI: 79.3-93.6%) in patients with CD4 ≤100 cells/μL, compared to only 56% for AlereLAM. When combined with sputum Xpert, 92% of all TB cases are detected in HIV-positive populations.
Implementation Evidence from Health Systems
India's RNTCP Demonstration Study (Sachdeva et al., 2015) tested 70,556 presumptive TB patients across 18 sub-district tuberculosis units, achieving a 39% increase in case notification (aIRR 1.39; 95% CI: 1.18-1.64) and 5-fold increase in rifampicin-resistant TB detection following Xpert decentralization to microscopy center level. Operational feasibility was high, with 99.1% valid results.
South Africa's national experience provides perhaps the most comprehensive implementation evidence. The Khayelitsha decentralization program (Cox et al., 2015) documented dramatic reductions in time-to-treatment: from 71 days (pre-program) to 28 days (decentralized care) to just 8 days (post-Xpert). The nationwide retrospective cohort (Cox et al., 2017; n=5,036) showed treatment initiation within 6 months improved from 55% to 63%, and pre-treatment mortality fell from 17.5% to 5.8% following Xpert implementation. The POC-based Lessells et al. (2017) cluster-randomized trial demonstrated point-of-care Xpert reduces median time-to-treatment to 1 day versus 7 days for laboratory-based testing.
Tanzania's MDR-TB program showed Xpert-based diagnosis reduced time-to-treatment from 155 days (phenotypic DST era) to 26 days (Xpert era) for drug-resistant cases.
Cost-Effectiveness and Financing
Economic evaluations consistently demonstrate cost-effectiveness at current pricing. In Mozambique, Orlando et al. (2018) calculated an incremental cost-effectiveness ratio of $56.54 per DALY averted for Xpert versus standard care in HIV-positive patients—well below the GDP-per-capita threshold. The Kenya LF-LAM analysis (Yakhelef et al., 2020) found even more favorable ratios: €4.6 (~$5) per DALY averted for LF-LAM-based algorithms in HIV-positive patients with CD4 <200.
Current per-test costs through Global Drug Facility negotiated pricing: Xpert MTB/RIF Ultra at $7.97, Truenat MTB Plus at $7.90, and AlereLAM at ~$3.50. Full operational costs including maintenance surcharges total approximately $20-26 per Xpert test at volumes of ≥3 tests daily.
Equity Impacts
Rapid diagnostics disproportionately benefit populations facing the greatest diagnostic delays. Only 7 of 30 high-burden countries report >50% of diagnostic sites with rapid test access (WHO, 2024). Rural populations experience 22-78% higher odds of diagnostic delay compared to urban populations. Pediatric TB presents unique challenges—78.9% of children under 5 years present with extrapulmonary TB, which has lower diagnostic yields. The systematic review by Naidoo et al. (2022) found molecular diagnostics reduce diagnostic delay by 40 days for drug-resistant TB compared to culture-based approaches.
Implementation Solution
Scalable Primary Care TB Diagnostic Program Framework
Site Selection Criteria: Prioritize facilities meeting ≥3 criteria: (1) TB case notification >50 annually; (2) HIV prevalence >5%; (3) distance >30 km from reference laboratory; (4) existing microscopy services; (5) stable electricity (or solar backup feasibility).
Test Selection Algorithm:
First-line for all presumptive TB: Xpert MTB/RIF Ultra (preferred) or Truenat MTB Plus (where infrastructure constraints exist)
HIV-positive patients with CD4 <200 or serious illness: Add urine LF-LAM (FujiLAM when available; AlereLAM currently)
Pediatric patients: Stool-based Xpert when respiratory specimens unavailable
Clinical Workflow Integration:
Day | Activity | Responsible Personnel | Output |
|---|---|---|---|
Day 0 | Patient presents with respiratory symptoms; clinician applies screening criteria (cough ≥2 weeks, fever, weight loss, night sweats, HIV status) | Clinical officer/nurse | Presumptive TB identification |
Day 0 | Sputum collection (spot specimen); urine collection if HIV+ | Nurse/CHW | Specimens for testing |
Day 0-1 | Rapid molecular test performed | Trained operator (lab tech/nurse) | Xpert/Truenat result |
Day 0-1 | Result recorded in TB register; clinician notified | Laboratory/data clerk | Documentation complete |
Day 1 | Positive result: Patient counseling, chest X-ray, treatment initiation | Clinical officer | TB treatment started |
Day 1-7 | Negative result with high clinical suspicion: Repeat testing, clinical diagnosis consideration | Clinical officer | Clinical pathway determination |
Day 7 | Rifampicin-resistant: Referral to DR-TB treatment site | Program coordinator | DR-TB care linkage |
Day 14-30 | Treatment adherence monitoring; contact tracing initiated | CHW/nurse | Treatment cascade support |
Staffing and Training Model:
Operators: 2 trained staff per site (primary + backup); 2-3 day competency-based training
Competency standards: ≥90% practical assessment, ≥80% written examination
Task-shifting: Evidence supports nurse and community health worker operation with equivalent outcomes to laboratory technicians (WHO Implementation Manual)
Quality Assurance Framework:
Monthly indicator monitoring: error rates (target <5%), invalid rates, turnaround time
Quarterly dried culture spot (DCS) proficiency testing panels via national TB reference laboratory
Annual module calibration ($450/4 modules) with service contract
Super-user network for basic troubleshooting
Data Systems:
Paper-based TB laboratory registers with digital backup
GxAlert or equivalent connectivity solution for automatic result transmission
Integration with national TB surveillance (e.g., DHIS2)
Dashboard for key performance indicators
Financing Pathway:
Year 1-2: Global Fund/PEPFAR catalytic funding for equipment and initial cartridge procurement via GDF
Year 2-3: Government budget line allocation for consumables (cartridges, maintenance)
Year 3+: Domestic financing with donor support for quality assurance and scale-up
Cost recovery not recommended given public health priority
Figure 1: Primary Care TB Diagnostic Pathway
Implementation Impact & Scalability
Projected Impact for a District of 500,000 Population
TB Case Detection (High-Burden Setting):
Using WHO incidence estimates and implementation study yields:

Metric | Conventional (Smear) | Rapid Molecular | Source |
|---|---|---|---|
Annual cases detected | 600-700 | 850-900 | Sachdeva et al., 2015 |
Bacteriological confirmation | 60% | 85% | India RNTCP data |
RR-TB detected | 15-20 | 75-100 | 5-fold increase documented |
Median diagnostic delay | 34 days | 2 days | Cox et al., 2015 |
Resource Requirements:
Sites requiring rapid diagnostics: 8-12 primary care facilities (based on TB burden distribution)
Health workers to train: 16-24 (2 per site); 2-3 days training each
Equipment cost: $140,000-228,000 (8-12 GeneXpert 4-module instruments at $17,500-19,000)
Annual cartridge cost: $160,000-180,000 (20,000 tests × $8)
Total Year 1 budget: $350,000-450,000; Years 2+: $180,000-220,000
Equity Impact: Rural TB detection rates projected to increase from 35% to 75% of expected cases with decentralization (WHO, 2024). Time-to-treatment for rifampicin-resistant TB reduced from 44 days to 8 days based on South African evidence (Cox et al., 2017).
Scalability: This model has been validated across India (70,556 patients), South Africa (>100,000 tests annually), and multiple East African settings. Key success factors include supply chain reliability, quality assurance systems, and treatment linkage protocols.
Evidence Gaps: Prospective trials comparing mortality outcomes between rapid molecular versus microscopy-based algorithms in primary care remain limited—the XTEND trial (Churchyard et al., 2015) found no mortality benefit despite improved diagnosis, highlighting the need for health systems strengthening beyond diagnostics alone.
References
Dorman SE, Schumacher SG, Alland D, et al. Xpert MTB/RIF Ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre diagnostic accuracy study. Lancet Infect Dis. 2018;18(1):76-84. https://doi.org/10.1016/S1473-3099(17)30691-6
Penn-Nicholson A, Gomathi SN, Engel N, et al. A prospective multicentre diagnostic accuracy study for the Truenat tuberculosis assays. Eur Respir J. 2021;58(5):2100526. https://doi.org/10.1183/13993003.00526-2021
Broger T, Nicol MP, Székely R, et al. Diagnostic accuracy of a novel tuberculosis point-of-care urine lipoarabinomannan assay for people living with HIV: A meta-analysis of individual in- and outpatient data. PLoS Med. 2020;17(5):e1003113. https://doi.org/10.1371/journal.pmed.1003113
Sachdeva KS, Raizada N, Sreenivas A, et al. Use of Xpert MTB/RIF in decentralized public health settings and its effect on pulmonary TB and DR-TB case finding in India. PLoS One. 2015;10(5):e0126065. https://doi.org/10.1371/journal.pone.0126065
Cox HS, Daniels JF, Muller O, et al. Impact of decentralized care and the Xpert MTB/RIF test on rifampicin-resistant tuberculosis treatment initiation in Khayelitsha, South Africa. Open Forum Infect Dis. 2015;2(1):ofv014. https://doi.org/10.1093/ofid/ofv014
Cox H, Dickson-Hall L, Ndjeka N, et al. Delays and loss to follow-up before treatment of drug-resistant tuberculosis following implementation of Xpert MTB/RIF in South Africa: a retrospective cohort study. PLoS Med. 2017;14(2):e1002238. https://doi.org/10.1371/journal.pmed.1002238
Churchyard GJ, Stevens WS, Mametja LD, et al. Xpert MTB/RIF versus sputum microscopy as the initial diagnostic test for tuberculosis: a cluster-randomised trial embedded in South African roll-out of Xpert MTB/RIF. Lancet Glob Health. 2015;3(8):e450-e457. https://doi.org/10.1016/S2214-109X(15)00100-X
Lessells RJ, Cooke GS, McGrath N, et al. Impact of point-of-care Xpert MTB/RIF on tuberculosis treatment initiation: a cluster-randomized trial. Am J Respir Crit Care Med. 2017;196(7):901-910. https://doi.org/10.1164/rccm.201702-0278OC
Orlando S, Triulzi I, Ciccacci F, et al. Delayed diagnosis and treatment of tuberculosis in HIV+ patients in Mozambique: a cost-effectiveness analysis of screening protocols based on four symptom screening, smear microscopy, urine LAM test and Xpert MTB/RIF. PLoS One. 2018;13(7):e0200523. https://doi.org/10.1371/journal.pone.0200523
Yakhelef N, Audibert M, Ferlazzo G, et al. Cost-effectiveness of diagnostic algorithms including lateral-flow urine lipoarabinomannan for HIV-positive patients with symptoms of tuberculosis in Kenya. PLoS One. 2020;15(1):e0227138. https://doi.org/10.1371/journal.pone.0227138
World Health Organization. Global Tuberculosis Report 2024. Geneva: WHO; 2024. https://www.who.int/teams/global-programme-on-tuberculosis-and-lung-health/tb-reports
Bjerrum S, Schiller I, Dendukuri N, et al. Lateral flow urine lipoarabinomannan assay for detecting active tuberculosis in people living with HIV. Cochrane Database Syst Rev. 2019;10(10):CD011420. https://doi.org/10.1002/14651858.CD011420.pub3
Turn evidence into everyday care.
No spam, unsubscribe anytime.