Preventing Postpartum Hemorrhage in Low-Resource Birth Settings
Verified by Sahaj Satani from ImplementMD


The Implementation Gap
Postpartum hemorrhage (PPH) causes approximately 14% of maternal deaths globally—an estimated 140,000 preventable deaths annually, with 95% occurring in low- and middle-income countries (LMICs). Active Management of the Third Stage of Labor (AMTSL)—comprising prophylactic uterotonic administration, controlled cord traction, and uterine massage—reduces PPH-related mortality by 60-66% (Begley et al., 2019). Yet WHO data reveals that fewer than 50% of births in low-income countries receive AMTSL, with baseline implementation rates as low as 3-5% in some African settings (POPPHI, 2007).
The implementation gap persists due to limited training infrastructure, oxytocin supply chain instability requiring cold-chain maintenance at 2-8°C, unclear clinical workflows for community settings, insufficient supervision systems, and poor integration into existing maternal health programs. This TIB provides an evidence-based implementation roadmap for scaling AMTSL through frontline health workers in district health systems.
Evidence for Implementation Readiness
Randomized Trial Evidence Demonstrates Strong AMTSL Efficacy
The WHO CHAMPION Trial (Widmer et al., 2018), the largest randomized controlled trial on uterotonics for PPH prevention, enrolled 29,645 women across 23 hospitals in 10 countries (Argentina, Egypt, India, Kenya, Nigeria, Singapore, South Africa, Thailand, Uganda, UK). New England Journal of Medicine This double-blind noninferiority trial compared heat-stable carbetocin (100μg IM) versus oxytocin (10 IU IM) immediately after vaginal birth. Results demonstrated noninferiority for the composite primary outcome of blood loss ≥500mL or additional uterotonic use: 14.5% vs 14.4% (RR 1.01; 95% CI 0.95-1.06). New England Journal of Medicine Critically, heat-stable carbetocin maintains efficacy at ≤30°C for three years, eliminating cold-chain barriers that compromise oxytocin quality in resource-limited settings. Dovepress
The landmark Cochrane systematic review comparing active versus expectant management (Begley et al., 2019), analyzing 8 trials with 8,892 women, found that AMTSL reduced severe PPH (≥1000mL) by 66% (RR 0.34; 95% CI 0.14-0.87) and maternal anemia (Hb <9 g/dL) by 50% (RR 0.50; 95% CI 0.30-0.83). The number needed to treat (NNT) to prevent one severe PPH was approximately 63 women receiving AMTSL.
The WOMAN Trial (WOMAN Trial Collaborators, 2017) demonstrated adjunctive benefit of tranexamic acid, randomizing 20,060 women with PPH across 193 hospitals in 21 countries. Death due to bleeding was reduced by 19% overall (RR 0.81; 95% CI 0.65-1.00; p=0.045), with 31% reduction when administered within 3 hours of birth (RR 0.69; 95% CI 0.52-0.91; p=0.008). Laparotomy to control bleeding decreased by 36% (RR 0.64; 95% CI 0.49-0.85; p=0.002).
Real-World Implementation Programs Show Scalability
The Helping Mothers Survive Bleeding After Birth (HMS BAB) cluster-randomized trial in Tanzania (Alwy Al-Beity et al., 2019) evaluated a competency-based training intervention across 61 facilities covering 120,533 deliveries. Using one-day simulation training with MamaNatalie® birthing simulators followed by 8 weeks of peer-led practice drills, the intervention achieved significant reductions in PPH-related near-misses (difference-in-differences: -5.3; 95% CI -7.8 to -2.7; p<0.001) and case fatality (difference-in-differences: -4.0; 95% CI -6.5 to -1.5; p<0.01). The cascade training model—master trainers to local facilitators to learners—proved effective across diverse rural facilities.
A Nigeria heat-stable carbetocin implementation study (Amode et al., 2024) demonstrated that with structured mentoring and supportive supervision, 56% of 18,364 deliveries received heat-stable carbetocin, reducing PPH incidence to 0.8%. ResearchGate Provider knowledge increased from 58% to 74% among physicians and 39% to 67% among nurse-midwives following in-service training combined with bi-weekly field supervision.
A systematic review and meta-analysis of AMTSL knowledge in Sub-Saharan Africa (Abebe Gelaw et al., 2024) found only 47.98% (95% CI 32.6-63.4%) of healthcare providers demonstrated adequate AMTSL knowledge. However, pre- and in-service training doubled the odds of adequate knowledge (AOR 2.25; 95% CI 1.00-5.08), and good practice experience increased odds nearly ninefold (AOR 8.91; 95% CI 4.58-17.40).
Cost-Effectiveness Supports Investment
The E-MOTIVE trial cost-effectiveness analysis (Williams et al., 2024) across 78 hospitals in Kenya, Nigeria, South Africa, and Tanzania found bundled PPH detection and treatment cost only $11.83 per severe PPH averted and $113.91 per DALY averted—far below the GDP-based threshold of $2,816. In South Africa, the intervention was cost-saving (dominant). Mean incremental cost was just $0.30 per patient.
In Vietnam, Tsu et al. (2009) found AMTSL with oxytocin ampoules added only $0.20 per woman to delivery costs, with cost per PPH case averted of $2.10 and cost per maternal death averted ranging from $7-2,508 depending on baseline PPH rates. In Senegal, community-based misoprostol cost $2.21 per delivery versus $4.38 for facility-based oxytocin (Vlassoff et al., 2016).
Equity Considerations Favor Task-Shifting Approaches
Task-shifting systematic reviews demonstrate that community health workers and traditional birth attendants can safely administer oral misoprostol (Spangler et al., 2018). A Pakistan RCT (Mobeen et al., 2011) showed TBA-administered misoprostol reduced PPH by 24% (RR 0.76; 95% CI 0.59-0.97). Community misoprostol distribution reaches 54.5-96.9% coverage when delivered during home visits in late pregnancy (Smith et al., 2013), with erroneous early administration occurring in only 0.06% of cases.
Implementation Solution
District-Level AMTSL Scale-Up Framework
Site Selection and Infrastructure Assessment
Implementation begins with mapping all birth delivery points within the district health system. Facilities are stratified by delivery volume and capability:
Facility Level | Birth Volume | AMTSL Components | Uterotonic Options |
|---|---|---|---|
District Hospital | >100/month | Full AMTSL + emergency management | Oxytocin IV/IM, carbetocin, TXA |
Primary Health Center | 20-100/month | Full AMTSL protocol | Oxytocin IM, misoprostol backup |
Community Health Post | <20/month | Modified AMTSL | Heat-stable carbetocin or misoprostol |
Home Births | Variable | Uterotonic prophylaxis | Oral misoprostol 600μg |
Cadre Training Strategy
Training requirements vary by provider cadre. Skilled birth attendants (midwives, nurse-midwives) receive comprehensive AMTSL training including injectable uterotonic administration, controlled cord traction, and complication recognition. Community health workers receive focused training on oral misoprostol distribution, danger sign recognition, and emergency referral pathways.
Training Curriculum Structure
Phase | Duration | Content | Assessment |
|---|---|---|---|
Classroom | 2 days | Third-stage physiology, AMTSL rationale, drug pharmacology, complication recognition | Written examination (≥80% pass) |
Simulation | 2 days | MamaNatalie® simulator practice, IM injection technique, uterine massage | Observed structured clinical examination |
Clinical Practicum | 2-4 weeks | Supervised deliveries (minimum 10 AMTSL-managed births) | Direct observation checklist |
Refresher | Quarterly | Skills update, case review, protocol reinforcement | Competency re-certification |
Workflow Redesign for Continuous PPH Prevention
Integration across the maternal care continuum ensures no opportunities are missed:
┌─────────────────────────────────────────────────────────────────────────────────────────┐ │ BIRTH CENTER PPH PREVENTION WORKFLOW │ │ Navy/Black Clinical Protocol │ ├─────────────────────────────────────────────────────────────────────────────────────────┤ │ │ │ ANTENATAL PERIOD INTRAPARTUM POSTPARTUM │ │ (Before delivery) (Third stage: 5-15 min) (0-24 hours) │ │ │ │ ┌──────────────┐ ┌──────────────┐ ┌──────────────────┐ ┌──────────────────┐ │ │ │ ANTENATAL │ │ LABOR │ │ ACTIVE MGMT OF │ │ POSTPARTUM │ │ │ │ CARE │───▶│ ADMISSION │───▶│ THIRD STAGE │───▶│ MONITORING │ │ │ │ │ │ │ │ │ │ │ │ │ │ • Risk ID │ │ • Partograph │ │ • Oxytocin 10 IU │ │ • Fundal tone │ │ │ │ • Hb check │ │ • IV access │ │ IM within 1 │ │ q15min × 2h │ │ │ │ • Blood type │ │ • Prepare │ │ minute of │ │ • Vital signs │ │ │ │ • Birth plan │ │ uterotonic │ │ delivery │ │ • Pad saturation │ │ │ │ • Danger │ │ │ │ • Controlled │ │ • Hb if bleeding │ │ │ │ sign ed. │ │ │ │ cord traction │ │ │ │ │ └──────────────┘ └──────────────┘ │ • Uterine │ └────────┬─────────┘ │ │ │ │ │ massage │ │ │ │ │ │ └────────┬─────────┘ │ │ │ │ │ │ │ │ │ ▼ ▼ ▼ ▼ │ │ ┌──────────────────────────────────────────────────────────────────────────────────┐ │ │ │ IF HEMORRHAGE DETECTED (>500mL) │ │ │ │ ┌─────────────┐ ┌─────────────┐ ┌─────────────┐ ┌─────────────────┐ │ │ │ │ │ Bimanual │───▶│ TXA 1g IV │───▶│ Additional │───▶│ EMERGENCY │ │ │ │ │ │ compression │ │ (within 3h) │ │ uterotonics │ │ REFERRAL │ │ │ │ │ │ │ │ │ │ │ │ • Blood bank │ │ │ │ │ │ │ │ │ │ │ │ • Surgical care │ │ │ │ │ └─────────────┘ └─────────────┘ └─────────────┘ └─────────────────┘ │ │ │ └──────────────────────────────────────────────────────────────────────────────────┘ │ │ │ │ TEMPORAL KEY: ━━━ Antenatal (weeks before) ━━━ Third stage (5-15 min) ━━━ Immediate │ │ postpartum │ └─────────────────────────────────────────────────────────────────────────────────────────┘
Drug Supply Chain Management
Oxytocin procurement must account for cold-chain requirements (2-8°C storage). Wiley Online LibraryFerring District pharmacies maintain 3-month buffer stocks with temperature monitoring logs. Heat-stable carbetocin offers a critical advantage—stability at ≤30°C for 3 years—making it preferable for peripheral facilities with unreliable electricity. DovepressFerring Misoprostol (600μg oral, room-temperature stable) serves as contingency when injectable uterotonics are unavailable or cold-chain is compromised. Wiley Online Library Monthly stock audits and automated reorder triggers prevent stockouts.
Quality Assurance System
Data registries track every delivery: uterotonic administered (type, timing, dose), estimated blood loss, PPH cases (≥500mL and ≥1000mL), referrals, and maternal deaths. Monthly facility-level dashboards display AMTSL coverage rates and PPH incidence. Supervision checklists completed quarterly assess partograph use, drug storage conditions, provider competency, and emergency readiness.
Financing Mechanisms
District budgets allocate dedicated line items for: uterotonic procurement ($0.27-0.31 per dose), Essentialmeds cold-chain equipment maintenance, training costs ($15-25 per provider trained), supervision transport, and data systems. RMNCAH (Reproductive, Maternal, Newborn, Child, and Adolescent Health) pooled funds and Global Financing Facility support provide transitional financing with explicit sustainability plans for government absorption within 3-5 years.
Implementation Impact and Scalability
Projected Outcomes for a 100,000-Birth District
For a district with 100,000 annual births, baseline PPH incidence of 10.5% (Sub-Saharan African average), and maternal mortality ratio of 400 per 100,000 live births, AMTSL scale-up generates substantial impact:
Mortality and Morbidity Reduction:
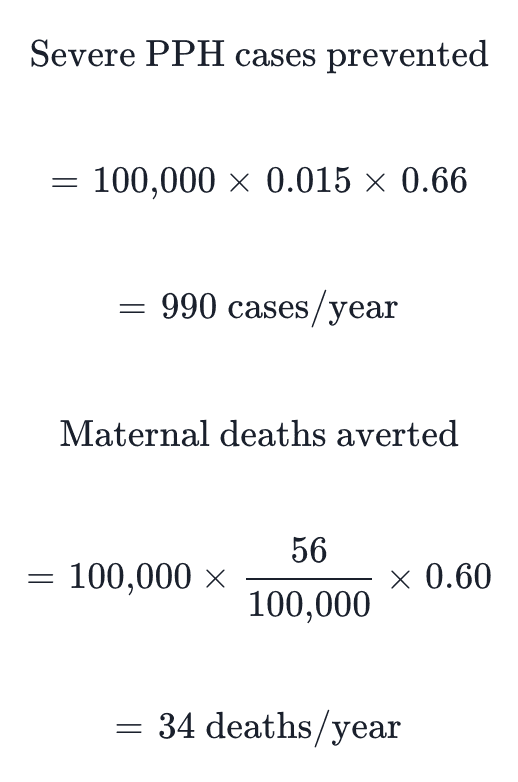
With 60-66% reduction in severe PPH through AMTSL implementation (Begley et al., 2019), approximately 990 severe hemorrhage cases and 34 maternal deaths would be prevented annually. At a cost of $113.91 per DALY averted (Williams et al., 2024), the intervention is highly cost-effective.
Implementation Requirements:
Health workers trained: 250-350 skilled birth attendants and community health workers
Training investment: $4,000-8,000 (initial) + $2,000/year (refresher)
Uterotonic supplies: $27,000-31,000 annually (at $0.27-0.31/dose)
Cold-chain and supervision: $5,000-10,000 annually
Scalability Assessment: The HMS BAB model demonstrates feasibility across diverse rural settings with minimal infrastructure requirements. Cascade training extends reach efficiently. Heat-stable carbetocin and misoprostol mitigate cold-chain barriers for the most remote facilities. Integration into existing antenatal care and skilled birth attendance programs maximizes sustainability.
Equity Impact: Community-based misoprostol distribution reaches 54.5-96.9% of home births (Smith et al., 2013), directly benefiting women in remote areas without facility access. Task-shifting to community health workers ensures the poorest quintiles—those with lowest facility delivery rates—receive PPH prophylaxis. PubMed Central
Evidence Gaps: Long-term sustainability data beyond 3-5 years remains limited. Optimal training dosage and frequency for competency retention requires further study. Heat-stable carbetocin effectiveness data in community (non-facility) settings is needed.
References
Abebe Gelaw, K., Kefale, B., Aregawi, G., Demis, S., & Gebremichael, B. (2024). Knowledge and factors associated with active management of the third stage of labor in sub-Saharan Africa: A systematic review and meta-analysis. International Journal of Gynecology & Obstetrics, 166(3), 943-953. https://doi.org/10.1002/ijgo.15560
Alwy Al-beity, F., Pembe, A. B., Hiber, A.,"; Hanson, C., et al. (2019). Effect of the competency-based Helping Mothers Survive Bleeding after Birth (HMS BAB) training on maternal morbidity: A cluster-randomised trial in 20 districts in Tanzania. BMJ Global Health, 4(2), e001214. https://doi.org/10.1136/bmjgh-2018-001214
Begley, C. M., Gyte, G. M. L., Devane, D., McGuire, W., Weeks, A., & Biesty, L. M. (2019). Active versus expectant management for women in the third stage of labour. Cochrane Database of Systematic Reviews, 2, CD007412. https://doi.org/10.1002/14651858.CD007412.pub5
Mobeen, N., Durocher, J., Zuberi, N., Jahan, N., Blum, J., Wasim, S., ... & Winikoff, B. (2011). Administration of misoprostol by trained traditional birth attendants to prevent postpartum haemorrhage in homebirths in Pakistan: A randomised placebo-controlled trial. BJOG: An International Journal of Obstetrics & Gynaecology, 118(3), 353-361. https://doi.org/10.1111/j.1471-0528.2010.02807.x
Smith, J. M., Gubin, R., Holston, M. M., Fullerton, J., & Prata, N. (2013). Misoprostol for postpartum hemorrhage prevention at home birth: An integrative review of global implementation experience to date. BMC Pregnancy and Childbirth, 13, 44. https://doi.org/10.1186/1471-2393-13-44
Spangler, S. A., & Patterson, J. (2018). Task shifting in active management of the third stage of labor: A systematic review. BMC Pregnancy and Childbirth, 18, 47. https://doi.org/10.1186/s12884-018-1677-5
Tsu, V. D., Luu, H. T., & Mai, T. T. (2009). Cost-effectiveness analysis of active management of third-stage labour in Vietnam. Health Policy and Planning, 24(6), 438-444. https://doi.org/10.1093/heapol/czp020
Vlassoff, M., Diallo, A., Philbin, J., Kost, K., & Bankole, A. (2016). Cost-effectiveness of two interventions for the prevention of postpartum hemorrhage in Senegal. International Journal of Gynecology & Obstetrics, 133(3), 307-311. https://doi.org/10.1016/j.ijgo.2015.10.015
Widmer, M., Piaggio, G., Nguyen, T. M. H., Osoti, A., Owa, O. O., Misra, S., ... & WHO CHAMPION Trial Group. (2018). Heat-stable carbetocin versus oxytocin to prevent hemorrhage after vaginal birth. New England Journal of Medicine, 379(8), 743-752. https://doi.org/10.1056/NEJMoa1805489
Williams, E. V., Vousden, N., Lawford, H. L. S., et al. (2024). A cost-effectiveness analysis of early detection and bundled treatment of postpartum hemorrhage alongside the E-MOTIVE trial. Nature Medicine, 30, 2343-2348. https://doi.org/10.1038/s41591-024-03069-5
WOMAN Trial Collaborators. (2017). Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): An international, randomised, double-blind, placebo-controlled trial. The Lancet, 389(10084), 2105-2116. https://doi.org/10.1016/S0140-6736(17)30638-4
The Implementation Gap
Postpartum hemorrhage (PPH) causes approximately 14% of maternal deaths globally—an estimated 140,000 preventable deaths annually, with 95% occurring in low- and middle-income countries (LMICs). Active Management of the Third Stage of Labor (AMTSL)—comprising prophylactic uterotonic administration, controlled cord traction, and uterine massage—reduces PPH-related mortality by 60-66% (Begley et al., 2019). Yet WHO data reveals that fewer than 50% of births in low-income countries receive AMTSL, with baseline implementation rates as low as 3-5% in some African settings (POPPHI, 2007).
The implementation gap persists due to limited training infrastructure, oxytocin supply chain instability requiring cold-chain maintenance at 2-8°C, unclear clinical workflows for community settings, insufficient supervision systems, and poor integration into existing maternal health programs. This TIB provides an evidence-based implementation roadmap for scaling AMTSL through frontline health workers in district health systems.
Evidence for Implementation Readiness
Randomized Trial Evidence Demonstrates Strong AMTSL Efficacy
The WHO CHAMPION Trial (Widmer et al., 2018), the largest randomized controlled trial on uterotonics for PPH prevention, enrolled 29,645 women across 23 hospitals in 10 countries (Argentina, Egypt, India, Kenya, Nigeria, Singapore, South Africa, Thailand, Uganda, UK). New England Journal of Medicine This double-blind noninferiority trial compared heat-stable carbetocin (100μg IM) versus oxytocin (10 IU IM) immediately after vaginal birth. Results demonstrated noninferiority for the composite primary outcome of blood loss ≥500mL or additional uterotonic use: 14.5% vs 14.4% (RR 1.01; 95% CI 0.95-1.06). New England Journal of Medicine Critically, heat-stable carbetocin maintains efficacy at ≤30°C for three years, eliminating cold-chain barriers that compromise oxytocin quality in resource-limited settings. Dovepress
The landmark Cochrane systematic review comparing active versus expectant management (Begley et al., 2019), analyzing 8 trials with 8,892 women, found that AMTSL reduced severe PPH (≥1000mL) by 66% (RR 0.34; 95% CI 0.14-0.87) and maternal anemia (Hb <9 g/dL) by 50% (RR 0.50; 95% CI 0.30-0.83). The number needed to treat (NNT) to prevent one severe PPH was approximately 63 women receiving AMTSL.
The WOMAN Trial (WOMAN Trial Collaborators, 2017) demonstrated adjunctive benefit of tranexamic acid, randomizing 20,060 women with PPH across 193 hospitals in 21 countries. Death due to bleeding was reduced by 19% overall (RR 0.81; 95% CI 0.65-1.00; p=0.045), with 31% reduction when administered within 3 hours of birth (RR 0.69; 95% CI 0.52-0.91; p=0.008). Laparotomy to control bleeding decreased by 36% (RR 0.64; 95% CI 0.49-0.85; p=0.002).
Real-World Implementation Programs Show Scalability
The Helping Mothers Survive Bleeding After Birth (HMS BAB) cluster-randomized trial in Tanzania (Alwy Al-Beity et al., 2019) evaluated a competency-based training intervention across 61 facilities covering 120,533 deliveries. Using one-day simulation training with MamaNatalie® birthing simulators followed by 8 weeks of peer-led practice drills, the intervention achieved significant reductions in PPH-related near-misses (difference-in-differences: -5.3; 95% CI -7.8 to -2.7; p<0.001) and case fatality (difference-in-differences: -4.0; 95% CI -6.5 to -1.5; p<0.01). The cascade training model—master trainers to local facilitators to learners—proved effective across diverse rural facilities.
A Nigeria heat-stable carbetocin implementation study (Amode et al., 2024) demonstrated that with structured mentoring and supportive supervision, 56% of 18,364 deliveries received heat-stable carbetocin, reducing PPH incidence to 0.8%. ResearchGate Provider knowledge increased from 58% to 74% among physicians and 39% to 67% among nurse-midwives following in-service training combined with bi-weekly field supervision.
A systematic review and meta-analysis of AMTSL knowledge in Sub-Saharan Africa (Abebe Gelaw et al., 2024) found only 47.98% (95% CI 32.6-63.4%) of healthcare providers demonstrated adequate AMTSL knowledge. However, pre- and in-service training doubled the odds of adequate knowledge (AOR 2.25; 95% CI 1.00-5.08), and good practice experience increased odds nearly ninefold (AOR 8.91; 95% CI 4.58-17.40).
Cost-Effectiveness Supports Investment
The E-MOTIVE trial cost-effectiveness analysis (Williams et al., 2024) across 78 hospitals in Kenya, Nigeria, South Africa, and Tanzania found bundled PPH detection and treatment cost only $11.83 per severe PPH averted and $113.91 per DALY averted—far below the GDP-based threshold of $2,816. In South Africa, the intervention was cost-saving (dominant). Mean incremental cost was just $0.30 per patient.
In Vietnam, Tsu et al. (2009) found AMTSL with oxytocin ampoules added only $0.20 per woman to delivery costs, with cost per PPH case averted of $2.10 and cost per maternal death averted ranging from $7-2,508 depending on baseline PPH rates. In Senegal, community-based misoprostol cost $2.21 per delivery versus $4.38 for facility-based oxytocin (Vlassoff et al., 2016).
Equity Considerations Favor Task-Shifting Approaches
Task-shifting systematic reviews demonstrate that community health workers and traditional birth attendants can safely administer oral misoprostol (Spangler et al., 2018). A Pakistan RCT (Mobeen et al., 2011) showed TBA-administered misoprostol reduced PPH by 24% (RR 0.76; 95% CI 0.59-0.97). Community misoprostol distribution reaches 54.5-96.9% coverage when delivered during home visits in late pregnancy (Smith et al., 2013), with erroneous early administration occurring in only 0.06% of cases.
Implementation Solution
District-Level AMTSL Scale-Up Framework
Site Selection and Infrastructure Assessment
Implementation begins with mapping all birth delivery points within the district health system. Facilities are stratified by delivery volume and capability:
Facility Level | Birth Volume | AMTSL Components | Uterotonic Options |
|---|---|---|---|
District Hospital | >100/month | Full AMTSL + emergency management | Oxytocin IV/IM, carbetocin, TXA |
Primary Health Center | 20-100/month | Full AMTSL protocol | Oxytocin IM, misoprostol backup |
Community Health Post | <20/month | Modified AMTSL | Heat-stable carbetocin or misoprostol |
Home Births | Variable | Uterotonic prophylaxis | Oral misoprostol 600μg |
Cadre Training Strategy
Training requirements vary by provider cadre. Skilled birth attendants (midwives, nurse-midwives) receive comprehensive AMTSL training including injectable uterotonic administration, controlled cord traction, and complication recognition. Community health workers receive focused training on oral misoprostol distribution, danger sign recognition, and emergency referral pathways.
Training Curriculum Structure
Phase | Duration | Content | Assessment |
|---|---|---|---|
Classroom | 2 days | Third-stage physiology, AMTSL rationale, drug pharmacology, complication recognition | Written examination (≥80% pass) |
Simulation | 2 days | MamaNatalie® simulator practice, IM injection technique, uterine massage | Observed structured clinical examination |
Clinical Practicum | 2-4 weeks | Supervised deliveries (minimum 10 AMTSL-managed births) | Direct observation checklist |
Refresher | Quarterly | Skills update, case review, protocol reinforcement | Competency re-certification |
Workflow Redesign for Continuous PPH Prevention
Integration across the maternal care continuum ensures no opportunities are missed:
┌─────────────────────────────────────────────────────────────────────────────────────────┐ │ BIRTH CENTER PPH PREVENTION WORKFLOW │ │ Navy/Black Clinical Protocol │ ├─────────────────────────────────────────────────────────────────────────────────────────┤ │ │ │ ANTENATAL PERIOD INTRAPARTUM POSTPARTUM │ │ (Before delivery) (Third stage: 5-15 min) (0-24 hours) │ │ │ │ ┌──────────────┐ ┌──────────────┐ ┌──────────────────┐ ┌──────────────────┐ │ │ │ ANTENATAL │ │ LABOR │ │ ACTIVE MGMT OF │ │ POSTPARTUM │ │ │ │ CARE │───▶│ ADMISSION │───▶│ THIRD STAGE │───▶│ MONITORING │ │ │ │ │ │ │ │ │ │ │ │ │ │ • Risk ID │ │ • Partograph │ │ • Oxytocin 10 IU │ │ • Fundal tone │ │ │ │ • Hb check │ │ • IV access │ │ IM within 1 │ │ q15min × 2h │ │ │ │ • Blood type │ │ • Prepare │ │ minute of │ │ • Vital signs │ │ │ │ • Birth plan │ │ uterotonic │ │ delivery │ │ • Pad saturation │ │ │ │ • Danger │ │ │ │ • Controlled │ │ • Hb if bleeding │ │ │ │ sign ed. │ │ │ │ cord traction │ │ │ │ │ └──────────────┘ └──────────────┘ │ • Uterine │ └────────┬─────────┘ │ │ │ │ │ massage │ │ │ │ │ │ └────────┬─────────┘ │ │ │ │ │ │ │ │ │ ▼ ▼ ▼ ▼ │ │ ┌──────────────────────────────────────────────────────────────────────────────────┐ │ │ │ IF HEMORRHAGE DETECTED (>500mL) │ │ │ │ ┌─────────────┐ ┌─────────────┐ ┌─────────────┐ ┌─────────────────┐ │ │ │ │ │ Bimanual │───▶│ TXA 1g IV │───▶│ Additional │───▶│ EMERGENCY │ │ │ │ │ │ compression │ │ (within 3h) │ │ uterotonics │ │ REFERRAL │ │ │ │ │ │ │ │ │ │ │ │ • Blood bank │ │ │ │ │ │ │ │ │ │ │ │ • Surgical care │ │ │ │ │ └─────────────┘ └─────────────┘ └─────────────┘ └─────────────────┘ │ │ │ └──────────────────────────────────────────────────────────────────────────────────┘ │ │ │ │ TEMPORAL KEY: ━━━ Antenatal (weeks before) ━━━ Third stage (5-15 min) ━━━ Immediate │ │ postpartum │ └─────────────────────────────────────────────────────────────────────────────────────────┘
Drug Supply Chain Management
Oxytocin procurement must account for cold-chain requirements (2-8°C storage). Wiley Online LibraryFerring District pharmacies maintain 3-month buffer stocks with temperature monitoring logs. Heat-stable carbetocin offers a critical advantage—stability at ≤30°C for 3 years—making it preferable for peripheral facilities with unreliable electricity. DovepressFerring Misoprostol (600μg oral, room-temperature stable) serves as contingency when injectable uterotonics are unavailable or cold-chain is compromised. Wiley Online Library Monthly stock audits and automated reorder triggers prevent stockouts.
Quality Assurance System
Data registries track every delivery: uterotonic administered (type, timing, dose), estimated blood loss, PPH cases (≥500mL and ≥1000mL), referrals, and maternal deaths. Monthly facility-level dashboards display AMTSL coverage rates and PPH incidence. Supervision checklists completed quarterly assess partograph use, drug storage conditions, provider competency, and emergency readiness.
Financing Mechanisms
District budgets allocate dedicated line items for: uterotonic procurement ($0.27-0.31 per dose), Essentialmeds cold-chain equipment maintenance, training costs ($15-25 per provider trained), supervision transport, and data systems. RMNCAH (Reproductive, Maternal, Newborn, Child, and Adolescent Health) pooled funds and Global Financing Facility support provide transitional financing with explicit sustainability plans for government absorption within 3-5 years.
Implementation Impact and Scalability
Projected Outcomes for a 100,000-Birth District
For a district with 100,000 annual births, baseline PPH incidence of 10.5% (Sub-Saharan African average), and maternal mortality ratio of 400 per 100,000 live births, AMTSL scale-up generates substantial impact:
Mortality and Morbidity Reduction:
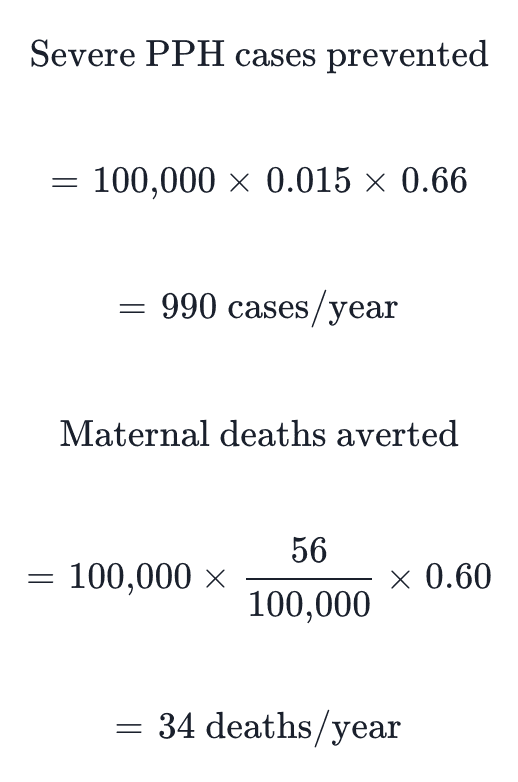
With 60-66% reduction in severe PPH through AMTSL implementation (Begley et al., 2019), approximately 990 severe hemorrhage cases and 34 maternal deaths would be prevented annually. At a cost of $113.91 per DALY averted (Williams et al., 2024), the intervention is highly cost-effective.
Implementation Requirements:
Health workers trained: 250-350 skilled birth attendants and community health workers
Training investment: $4,000-8,000 (initial) + $2,000/year (refresher)
Uterotonic supplies: $27,000-31,000 annually (at $0.27-0.31/dose)
Cold-chain and supervision: $5,000-10,000 annually
Scalability Assessment: The HMS BAB model demonstrates feasibility across diverse rural settings with minimal infrastructure requirements. Cascade training extends reach efficiently. Heat-stable carbetocin and misoprostol mitigate cold-chain barriers for the most remote facilities. Integration into existing antenatal care and skilled birth attendance programs maximizes sustainability.
Equity Impact: Community-based misoprostol distribution reaches 54.5-96.9% of home births (Smith et al., 2013), directly benefiting women in remote areas without facility access. Task-shifting to community health workers ensures the poorest quintiles—those with lowest facility delivery rates—receive PPH prophylaxis. PubMed Central
Evidence Gaps: Long-term sustainability data beyond 3-5 years remains limited. Optimal training dosage and frequency for competency retention requires further study. Heat-stable carbetocin effectiveness data in community (non-facility) settings is needed.
References
Abebe Gelaw, K., Kefale, B., Aregawi, G., Demis, S., & Gebremichael, B. (2024). Knowledge and factors associated with active management of the third stage of labor in sub-Saharan Africa: A systematic review and meta-analysis. International Journal of Gynecology & Obstetrics, 166(3), 943-953. https://doi.org/10.1002/ijgo.15560
Alwy Al-beity, F., Pembe, A. B., Hiber, A.,"; Hanson, C., et al. (2019). Effect of the competency-based Helping Mothers Survive Bleeding after Birth (HMS BAB) training on maternal morbidity: A cluster-randomised trial in 20 districts in Tanzania. BMJ Global Health, 4(2), e001214. https://doi.org/10.1136/bmjgh-2018-001214
Begley, C. M., Gyte, G. M. L., Devane, D., McGuire, W., Weeks, A., & Biesty, L. M. (2019). Active versus expectant management for women in the third stage of labour. Cochrane Database of Systematic Reviews, 2, CD007412. https://doi.org/10.1002/14651858.CD007412.pub5
Mobeen, N., Durocher, J., Zuberi, N., Jahan, N., Blum, J., Wasim, S., ... & Winikoff, B. (2011). Administration of misoprostol by trained traditional birth attendants to prevent postpartum haemorrhage in homebirths in Pakistan: A randomised placebo-controlled trial. BJOG: An International Journal of Obstetrics & Gynaecology, 118(3), 353-361. https://doi.org/10.1111/j.1471-0528.2010.02807.x
Smith, J. M., Gubin, R., Holston, M. M., Fullerton, J., & Prata, N. (2013). Misoprostol for postpartum hemorrhage prevention at home birth: An integrative review of global implementation experience to date. BMC Pregnancy and Childbirth, 13, 44. https://doi.org/10.1186/1471-2393-13-44
Spangler, S. A., & Patterson, J. (2018). Task shifting in active management of the third stage of labor: A systematic review. BMC Pregnancy and Childbirth, 18, 47. https://doi.org/10.1186/s12884-018-1677-5
Tsu, V. D., Luu, H. T., & Mai, T. T. (2009). Cost-effectiveness analysis of active management of third-stage labour in Vietnam. Health Policy and Planning, 24(6), 438-444. https://doi.org/10.1093/heapol/czp020
Vlassoff, M., Diallo, A., Philbin, J., Kost, K., & Bankole, A. (2016). Cost-effectiveness of two interventions for the prevention of postpartum hemorrhage in Senegal. International Journal of Gynecology & Obstetrics, 133(3), 307-311. https://doi.org/10.1016/j.ijgo.2015.10.015
Widmer, M., Piaggio, G., Nguyen, T. M. H., Osoti, A., Owa, O. O., Misra, S., ... & WHO CHAMPION Trial Group. (2018). Heat-stable carbetocin versus oxytocin to prevent hemorrhage after vaginal birth. New England Journal of Medicine, 379(8), 743-752. https://doi.org/10.1056/NEJMoa1805489
Williams, E. V., Vousden, N., Lawford, H. L. S., et al. (2024). A cost-effectiveness analysis of early detection and bundled treatment of postpartum hemorrhage alongside the E-MOTIVE trial. Nature Medicine, 30, 2343-2348. https://doi.org/10.1038/s41591-024-03069-5
WOMAN Trial Collaborators. (2017). Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): An international, randomised, double-blind, placebo-controlled trial. The Lancet, 389(10084), 2105-2116. https://doi.org/10.1016/S0140-6736(17)30638-4
The Implementation Gap
Postpartum hemorrhage (PPH) causes approximately 14% of maternal deaths globally—an estimated 140,000 preventable deaths annually, with 95% occurring in low- and middle-income countries (LMICs). Active Management of the Third Stage of Labor (AMTSL)—comprising prophylactic uterotonic administration, controlled cord traction, and uterine massage—reduces PPH-related mortality by 60-66% (Begley et al., 2019). Yet WHO data reveals that fewer than 50% of births in low-income countries receive AMTSL, with baseline implementation rates as low as 3-5% in some African settings (POPPHI, 2007).
The implementation gap persists due to limited training infrastructure, oxytocin supply chain instability requiring cold-chain maintenance at 2-8°C, unclear clinical workflows for community settings, insufficient supervision systems, and poor integration into existing maternal health programs. This TIB provides an evidence-based implementation roadmap for scaling AMTSL through frontline health workers in district health systems.
Evidence for Implementation Readiness
Randomized Trial Evidence Demonstrates Strong AMTSL Efficacy
The WHO CHAMPION Trial (Widmer et al., 2018), the largest randomized controlled trial on uterotonics for PPH prevention, enrolled 29,645 women across 23 hospitals in 10 countries (Argentina, Egypt, India, Kenya, Nigeria, Singapore, South Africa, Thailand, Uganda, UK). New England Journal of Medicine This double-blind noninferiority trial compared heat-stable carbetocin (100μg IM) versus oxytocin (10 IU IM) immediately after vaginal birth. Results demonstrated noninferiority for the composite primary outcome of blood loss ≥500mL or additional uterotonic use: 14.5% vs 14.4% (RR 1.01; 95% CI 0.95-1.06). New England Journal of Medicine Critically, heat-stable carbetocin maintains efficacy at ≤30°C for three years, eliminating cold-chain barriers that compromise oxytocin quality in resource-limited settings. Dovepress
The landmark Cochrane systematic review comparing active versus expectant management (Begley et al., 2019), analyzing 8 trials with 8,892 women, found that AMTSL reduced severe PPH (≥1000mL) by 66% (RR 0.34; 95% CI 0.14-0.87) and maternal anemia (Hb <9 g/dL) by 50% (RR 0.50; 95% CI 0.30-0.83). The number needed to treat (NNT) to prevent one severe PPH was approximately 63 women receiving AMTSL.
The WOMAN Trial (WOMAN Trial Collaborators, 2017) demonstrated adjunctive benefit of tranexamic acid, randomizing 20,060 women with PPH across 193 hospitals in 21 countries. Death due to bleeding was reduced by 19% overall (RR 0.81; 95% CI 0.65-1.00; p=0.045), with 31% reduction when administered within 3 hours of birth (RR 0.69; 95% CI 0.52-0.91; p=0.008). Laparotomy to control bleeding decreased by 36% (RR 0.64; 95% CI 0.49-0.85; p=0.002).
Real-World Implementation Programs Show Scalability
The Helping Mothers Survive Bleeding After Birth (HMS BAB) cluster-randomized trial in Tanzania (Alwy Al-Beity et al., 2019) evaluated a competency-based training intervention across 61 facilities covering 120,533 deliveries. Using one-day simulation training with MamaNatalie® birthing simulators followed by 8 weeks of peer-led practice drills, the intervention achieved significant reductions in PPH-related near-misses (difference-in-differences: -5.3; 95% CI -7.8 to -2.7; p<0.001) and case fatality (difference-in-differences: -4.0; 95% CI -6.5 to -1.5; p<0.01). The cascade training model—master trainers to local facilitators to learners—proved effective across diverse rural facilities.
A Nigeria heat-stable carbetocin implementation study (Amode et al., 2024) demonstrated that with structured mentoring and supportive supervision, 56% of 18,364 deliveries received heat-stable carbetocin, reducing PPH incidence to 0.8%. ResearchGate Provider knowledge increased from 58% to 74% among physicians and 39% to 67% among nurse-midwives following in-service training combined with bi-weekly field supervision.
A systematic review and meta-analysis of AMTSL knowledge in Sub-Saharan Africa (Abebe Gelaw et al., 2024) found only 47.98% (95% CI 32.6-63.4%) of healthcare providers demonstrated adequate AMTSL knowledge. However, pre- and in-service training doubled the odds of adequate knowledge (AOR 2.25; 95% CI 1.00-5.08), and good practice experience increased odds nearly ninefold (AOR 8.91; 95% CI 4.58-17.40).
Cost-Effectiveness Supports Investment
The E-MOTIVE trial cost-effectiveness analysis (Williams et al., 2024) across 78 hospitals in Kenya, Nigeria, South Africa, and Tanzania found bundled PPH detection and treatment cost only $11.83 per severe PPH averted and $113.91 per DALY averted—far below the GDP-based threshold of $2,816. In South Africa, the intervention was cost-saving (dominant). Mean incremental cost was just $0.30 per patient.
In Vietnam, Tsu et al. (2009) found AMTSL with oxytocin ampoules added only $0.20 per woman to delivery costs, with cost per PPH case averted of $2.10 and cost per maternal death averted ranging from $7-2,508 depending on baseline PPH rates. In Senegal, community-based misoprostol cost $2.21 per delivery versus $4.38 for facility-based oxytocin (Vlassoff et al., 2016).
Equity Considerations Favor Task-Shifting Approaches
Task-shifting systematic reviews demonstrate that community health workers and traditional birth attendants can safely administer oral misoprostol (Spangler et al., 2018). A Pakistan RCT (Mobeen et al., 2011) showed TBA-administered misoprostol reduced PPH by 24% (RR 0.76; 95% CI 0.59-0.97). Community misoprostol distribution reaches 54.5-96.9% coverage when delivered during home visits in late pregnancy (Smith et al., 2013), with erroneous early administration occurring in only 0.06% of cases.
Implementation Solution
District-Level AMTSL Scale-Up Framework
Site Selection and Infrastructure Assessment
Implementation begins with mapping all birth delivery points within the district health system. Facilities are stratified by delivery volume and capability:
Facility Level | Birth Volume | AMTSL Components | Uterotonic Options |
|---|---|---|---|
District Hospital | >100/month | Full AMTSL + emergency management | Oxytocin IV/IM, carbetocin, TXA |
Primary Health Center | 20-100/month | Full AMTSL protocol | Oxytocin IM, misoprostol backup |
Community Health Post | <20/month | Modified AMTSL | Heat-stable carbetocin or misoprostol |
Home Births | Variable | Uterotonic prophylaxis | Oral misoprostol 600μg |
Cadre Training Strategy
Training requirements vary by provider cadre. Skilled birth attendants (midwives, nurse-midwives) receive comprehensive AMTSL training including injectable uterotonic administration, controlled cord traction, and complication recognition. Community health workers receive focused training on oral misoprostol distribution, danger sign recognition, and emergency referral pathways.
Training Curriculum Structure
Phase | Duration | Content | Assessment |
|---|---|---|---|
Classroom | 2 days | Third-stage physiology, AMTSL rationale, drug pharmacology, complication recognition | Written examination (≥80% pass) |
Simulation | 2 days | MamaNatalie® simulator practice, IM injection technique, uterine massage | Observed structured clinical examination |
Clinical Practicum | 2-4 weeks | Supervised deliveries (minimum 10 AMTSL-managed births) | Direct observation checklist |
Refresher | Quarterly | Skills update, case review, protocol reinforcement | Competency re-certification |
Workflow Redesign for Continuous PPH Prevention
Integration across the maternal care continuum ensures no opportunities are missed:
┌─────────────────────────────────────────────────────────────────────────────────────────┐ │ BIRTH CENTER PPH PREVENTION WORKFLOW │ │ Navy/Black Clinical Protocol │ ├─────────────────────────────────────────────────────────────────────────────────────────┤ │ │ │ ANTENATAL PERIOD INTRAPARTUM POSTPARTUM │ │ (Before delivery) (Third stage: 5-15 min) (0-24 hours) │ │ │ │ ┌──────────────┐ ┌──────────────┐ ┌──────────────────┐ ┌──────────────────┐ │ │ │ ANTENATAL │ │ LABOR │ │ ACTIVE MGMT OF │ │ POSTPARTUM │ │ │ │ CARE │───▶│ ADMISSION │───▶│ THIRD STAGE │───▶│ MONITORING │ │ │ │ │ │ │ │ │ │ │ │ │ │ • Risk ID │ │ • Partograph │ │ • Oxytocin 10 IU │ │ • Fundal tone │ │ │ │ • Hb check │ │ • IV access │ │ IM within 1 │ │ q15min × 2h │ │ │ │ • Blood type │ │ • Prepare │ │ minute of │ │ • Vital signs │ │ │ │ • Birth plan │ │ uterotonic │ │ delivery │ │ • Pad saturation │ │ │ │ • Danger │ │ │ │ • Controlled │ │ • Hb if bleeding │ │ │ │ sign ed. │ │ │ │ cord traction │ │ │ │ │ └──────────────┘ └──────────────┘ │ • Uterine │ └────────┬─────────┘ │ │ │ │ │ massage │ │ │ │ │ │ └────────┬─────────┘ │ │ │ │ │ │ │ │ │ ▼ ▼ ▼ ▼ │ │ ┌──────────────────────────────────────────────────────────────────────────────────┐ │ │ │ IF HEMORRHAGE DETECTED (>500mL) │ │ │ │ ┌─────────────┐ ┌─────────────┐ ┌─────────────┐ ┌─────────────────┐ │ │ │ │ │ Bimanual │───▶│ TXA 1g IV │───▶│ Additional │───▶│ EMERGENCY │ │ │ │ │ │ compression │ │ (within 3h) │ │ uterotonics │ │ REFERRAL │ │ │ │ │ │ │ │ │ │ │ │ • Blood bank │ │ │ │ │ │ │ │ │ │ │ │ • Surgical care │ │ │ │ │ └─────────────┘ └─────────────┘ └─────────────┘ └─────────────────┘ │ │ │ └──────────────────────────────────────────────────────────────────────────────────┘ │ │ │ │ TEMPORAL KEY: ━━━ Antenatal (weeks before) ━━━ Third stage (5-15 min) ━━━ Immediate │ │ postpartum │ └─────────────────────────────────────────────────────────────────────────────────────────┘
Drug Supply Chain Management
Oxytocin procurement must account for cold-chain requirements (2-8°C storage). Wiley Online LibraryFerring District pharmacies maintain 3-month buffer stocks with temperature monitoring logs. Heat-stable carbetocin offers a critical advantage—stability at ≤30°C for 3 years—making it preferable for peripheral facilities with unreliable electricity. DovepressFerring Misoprostol (600μg oral, room-temperature stable) serves as contingency when injectable uterotonics are unavailable or cold-chain is compromised. Wiley Online Library Monthly stock audits and automated reorder triggers prevent stockouts.
Quality Assurance System
Data registries track every delivery: uterotonic administered (type, timing, dose), estimated blood loss, PPH cases (≥500mL and ≥1000mL), referrals, and maternal deaths. Monthly facility-level dashboards display AMTSL coverage rates and PPH incidence. Supervision checklists completed quarterly assess partograph use, drug storage conditions, provider competency, and emergency readiness.
Financing Mechanisms
District budgets allocate dedicated line items for: uterotonic procurement ($0.27-0.31 per dose), Essentialmeds cold-chain equipment maintenance, training costs ($15-25 per provider trained), supervision transport, and data systems. RMNCAH (Reproductive, Maternal, Newborn, Child, and Adolescent Health) pooled funds and Global Financing Facility support provide transitional financing with explicit sustainability plans for government absorption within 3-5 years.
Implementation Impact and Scalability
Projected Outcomes for a 100,000-Birth District
For a district with 100,000 annual births, baseline PPH incidence of 10.5% (Sub-Saharan African average), and maternal mortality ratio of 400 per 100,000 live births, AMTSL scale-up generates substantial impact:
Mortality and Morbidity Reduction:
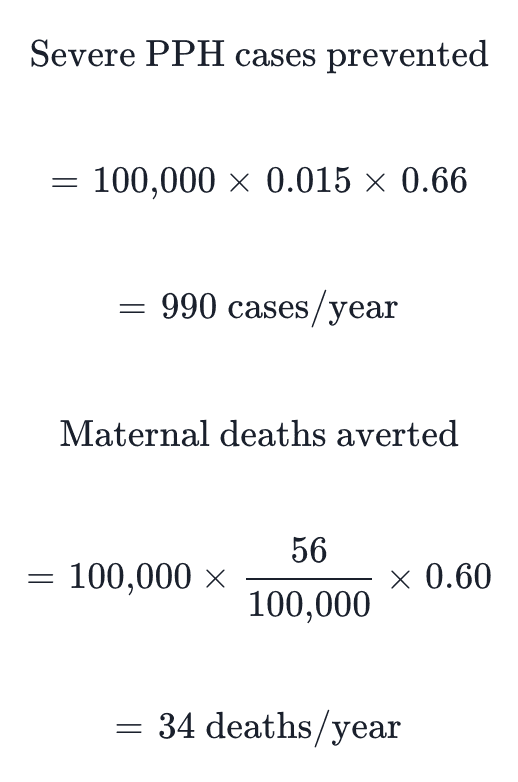
With 60-66% reduction in severe PPH through AMTSL implementation (Begley et al., 2019), approximately 990 severe hemorrhage cases and 34 maternal deaths would be prevented annually. At a cost of $113.91 per DALY averted (Williams et al., 2024), the intervention is highly cost-effective.
Implementation Requirements:
Health workers trained: 250-350 skilled birth attendants and community health workers
Training investment: $4,000-8,000 (initial) + $2,000/year (refresher)
Uterotonic supplies: $27,000-31,000 annually (at $0.27-0.31/dose)
Cold-chain and supervision: $5,000-10,000 annually
Scalability Assessment: The HMS BAB model demonstrates feasibility across diverse rural settings with minimal infrastructure requirements. Cascade training extends reach efficiently. Heat-stable carbetocin and misoprostol mitigate cold-chain barriers for the most remote facilities. Integration into existing antenatal care and skilled birth attendance programs maximizes sustainability.
Equity Impact: Community-based misoprostol distribution reaches 54.5-96.9% of home births (Smith et al., 2013), directly benefiting women in remote areas without facility access. Task-shifting to community health workers ensures the poorest quintiles—those with lowest facility delivery rates—receive PPH prophylaxis. PubMed Central
Evidence Gaps: Long-term sustainability data beyond 3-5 years remains limited. Optimal training dosage and frequency for competency retention requires further study. Heat-stable carbetocin effectiveness data in community (non-facility) settings is needed.
References
Abebe Gelaw, K., Kefale, B., Aregawi, G., Demis, S., & Gebremichael, B. (2024). Knowledge and factors associated with active management of the third stage of labor in sub-Saharan Africa: A systematic review and meta-analysis. International Journal of Gynecology & Obstetrics, 166(3), 943-953. https://doi.org/10.1002/ijgo.15560
Alwy Al-beity, F., Pembe, A. B., Hiber, A.,"; Hanson, C., et al. (2019). Effect of the competency-based Helping Mothers Survive Bleeding after Birth (HMS BAB) training on maternal morbidity: A cluster-randomised trial in 20 districts in Tanzania. BMJ Global Health, 4(2), e001214. https://doi.org/10.1136/bmjgh-2018-001214
Begley, C. M., Gyte, G. M. L., Devane, D., McGuire, W., Weeks, A., & Biesty, L. M. (2019). Active versus expectant management for women in the third stage of labour. Cochrane Database of Systematic Reviews, 2, CD007412. https://doi.org/10.1002/14651858.CD007412.pub5
Mobeen, N., Durocher, J., Zuberi, N., Jahan, N., Blum, J., Wasim, S., ... & Winikoff, B. (2011). Administration of misoprostol by trained traditional birth attendants to prevent postpartum haemorrhage in homebirths in Pakistan: A randomised placebo-controlled trial. BJOG: An International Journal of Obstetrics & Gynaecology, 118(3), 353-361. https://doi.org/10.1111/j.1471-0528.2010.02807.x
Smith, J. M., Gubin, R., Holston, M. M., Fullerton, J., & Prata, N. (2013). Misoprostol for postpartum hemorrhage prevention at home birth: An integrative review of global implementation experience to date. BMC Pregnancy and Childbirth, 13, 44. https://doi.org/10.1186/1471-2393-13-44
Spangler, S. A., & Patterson, J. (2018). Task shifting in active management of the third stage of labor: A systematic review. BMC Pregnancy and Childbirth, 18, 47. https://doi.org/10.1186/s12884-018-1677-5
Tsu, V. D., Luu, H. T., & Mai, T. T. (2009). Cost-effectiveness analysis of active management of third-stage labour in Vietnam. Health Policy and Planning, 24(6), 438-444. https://doi.org/10.1093/heapol/czp020
Vlassoff, M., Diallo, A., Philbin, J., Kost, K., & Bankole, A. (2016). Cost-effectiveness of two interventions for the prevention of postpartum hemorrhage in Senegal. International Journal of Gynecology & Obstetrics, 133(3), 307-311. https://doi.org/10.1016/j.ijgo.2015.10.015
Widmer, M., Piaggio, G., Nguyen, T. M. H., Osoti, A., Owa, O. O., Misra, S., ... & WHO CHAMPION Trial Group. (2018). Heat-stable carbetocin versus oxytocin to prevent hemorrhage after vaginal birth. New England Journal of Medicine, 379(8), 743-752. https://doi.org/10.1056/NEJMoa1805489
Williams, E. V., Vousden, N., Lawford, H. L. S., et al. (2024). A cost-effectiveness analysis of early detection and bundled treatment of postpartum hemorrhage alongside the E-MOTIVE trial. Nature Medicine, 30, 2343-2348. https://doi.org/10.1038/s41591-024-03069-5
WOMAN Trial Collaborators. (2017). Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): An international, randomised, double-blind, placebo-controlled trial. The Lancet, 389(10084), 2105-2116. https://doi.org/10.1016/S0140-6736(17)30638-4
Turn evidence into everyday care.
No spam, unsubscribe anytime.